Summary: A groundbreaking clinical study shows that closed-loop vagus nerve stimulation (CLV), paired with rehabilitation, can significantly improve arm and hand function in people with chronic spinal cord injuries. The therapy uses a tiny implanted device that sends timed electrical pulses to the brain when patients perform successful movements, helping rewire neural circuits.
Participants who previously saw no benefit from therapy alone experienced unprecedented recovery through CLV, regardless of age, injury duration, or severity. These results mark a major step toward potential FDA approval and offer hope to patients with no current treatment options.
Key Facts:
- Breakthrough Recovery: CLV led to major functional gains in upper limbs for spinal cord injury patients.
- Technology: A tiny implant sends electrical pulses during targeted movement therapy.
- Next Step: A Phase 3 trial with 70 patients will test CLV’s efficacy for FDA approval.
Source: UT Dallas
In a new clinical study, researchers from the Texas Biomedical Device Center (TxBDC) at The University of Texas at Dallas demonstrated unprecedented rates of recovery for spinal cord injuries.
In this study, published in the prestigious journal Nature on May 21, individuals with incomplete spinal cord injury safely received a combination of stimulation of a nerve in the neck with progressive, individualized rehabilitation.
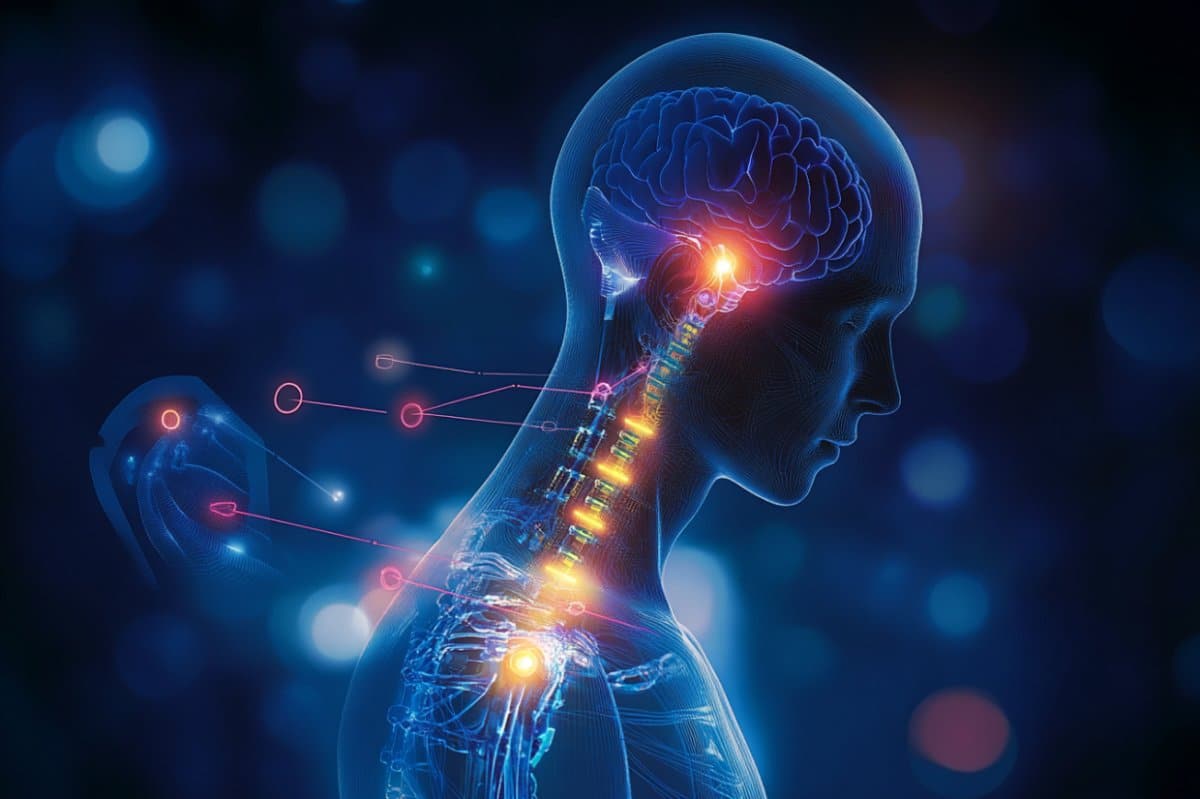
This approach, called closed-loop vagus nerve stimulation (CLV), produced meaningful improvements in arm and hand function in these individuals.
The unprecedented results position the UT Dallas scientists to proceed with a pivotal trial — the final hurdle on the road to potential Food and Drug Administration (FDA) approval of vagus nerve stimulation for treatment of upper-limb impairment due to spinal cord injury.
This approach is based on over a decade of neuroscience and bioengineering efforts by investigators at UT Dallas. The therapy uses electrical pulses sent to the brain via a tiny device implanted in the neck and timed to occur during rehabilitative exercises.
Previous work by UT Dallas researchers has demonstrated that stimulating the vagus nerve during physical therapy can rewire areas of the brain damaged by stroke and lead to improved recovery.
Dr. Michael Kilgard, the Margaret Fonde Jonsson Professor of neuroscience in the School of Behavioral and Brain Sciences and corresponding author, explained that treating spinal cord injury with CLV is different than conditions targeted in earlier studies.
“In stroke, people who do only therapy may get better, and adding CLV multiplies that improvement,” he said. “This study is different: Therapy alone for spinal cord injury didn’t help our participants at all.”
The trial involved 19 participants with chronic, incomplete cervical spinal cord injury. Each person performed 12 weeks of therapy, playing simple video games to trigger specific upper-limb movements. The implant was activated upon successful movements, resulting in significant benefits for arm and hand strength.
“These activities allow patients to regain strength, speed, range of motion and hand function. They simplify daily living,” said Dr. Robert Rennaker, professor of neuroscience and the Texas Instruments Distinguished Chair in Bioengineering, who designed the miniature implanted CLV device.
The study served as both a Phase 1 and Phase 2 clinical trial and included randomized placebo control in its first phase, in which nine of the 19 participants received sham stimulation rather than active treatment during the first 18 therapy sessions, then received CLV in the latter 18 sessions.
The participants ranged in age from 21 to 65 and were from one to 45 years post-injury. Neither of those factors, nor the severity of the impairment in those with any hand movement, influenced the degree of response to treatment.
“This approach produces results regardless of these factors, which often cause significant differences in success rates of other types of treatment,” said study co-author Dr. Jane Wigginton, medical doctor and chief medical officer at TxBDC, co-director of UTD’s Clinical and Translational Research Center, and medical science research director at the Center for BrainHealth.
“It is remarkable from a medical standpoint,” said Wigginton, who planned the clinical interactions and patient protections for the trial.
TxBDC has worked to treat a wide variety of conditions using CLV across 13 years of research. As a result, the FDA has approved vagus nerve stimulation for treating impaired upper-limb movement in stroke patients.
Wigginton said the latest results are especially exciting because they help people for whom there is no existing solution.
“The people in this study have now gained the ability to do things that are meaningful for them and impactful in their lives.”
The newest generation of the implantable CLV device, designed by Rennaker, is approximately 50 times smaller than their version from three years ago. It does not prevent patients from receiving MRIs, CT scans or ultrasounds.
A Phase 3 pivotal trial will include 70 participants at multiple U.S. institutions that specialize in spinal cord injury.
Co-author Dr. Seth Hays, associate professor of bioengineering and Fellow, Eugene McDermott Distinguished Professor in the Erik Jonsson School of Engineering and Computer Science, has been with the CLV project dating back to the earliest studies.
“Prior to this study, no person with spinal cord injury had ever received CLV,” he said. “This is the first evidence that gains can be made. Now we will set about determining how we make this optimally effective.”
Hays cautioned that it is not a foregone conclusion that the therapy will make it to patients after the next trial.
“We still have a long road ahead. For many reasons — financial, regulatory or scientific — this could still die on the vine,” he said. “But we have positioned ourselves to succeed.”
The research team emphasized the importance of the dozens of people involved in the work — both the patients and TxBDC’s partners at Baylor University Medical Center, Baylor Scott & White Research Institute and Baylor Scott & White Institute for Rehabilitation.
“This has been the hardest working, most altruistic group of professionals, and that has been incredibly impactful,” Wigginton said.
Noting that even outpatient surgery is complex for those with impaired mobility, Rennaker added, “These patients said, ‘Put that device in me’ — that’s a huge commitment. They deserve credit for paving the path for others.”
Other UTD-affiliated co-authors included Joseph Epperson BS’20, PhD’24, TxBDC research associate; cognition and neuroscience doctoral student Emmanuel Adehunoluwa MS’23; Amy Porter MBA’20, TxBDC director of operations; Holle Carey Gallaway MBA’23, TxBDC research biomedical engineer; and David Pruitt MS’14, PhD’16.
Kilgard has a financial interest in MicroTransponder Inc., which markets vagus nerve stimulation therapy for stroke. Rennaker is the founder and CEO of XNerve, which developed the device used in this study.
Funding: The research was funded by a grant (N66001-17-2-4011) from the Defense Advanced Research Projects Agency (DARPA), an agency of the Department of Defense, as well as the Wings for Life Accelerated Translational Program.
About this neurotech and SCI research news
Author: Stephen Fontenot
Source: UT Dallas
Contact: Stephen Fontenot – UT Dallas
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Closed-loop vagus nerve stimulation aids recovery from spinal cord injury” by Michael Kilgard et al. Nature
Abstract
Closed-loop vagus nerve stimulation aids recovery from spinal cord injury
Decades of research have demonstrated that recovery from serious neurological injury will require synergistic therapeutic approaches. Rewiring spared neural circuits after injury is a long-standing goal of neurorehabilitation.
We hypothesized that combining intensive, progressive, task-focused training with real-time closed-loop vagus nerve stimulation (CLV) to enhance synaptic plasticity could increase strength, expand range of motion and improve hand function in people with chronic, incomplete cervical spinal cord injury.
Here we report the results from a prospective, double-blinded, sham-controlled, randomized study combining gamified physical therapy using force and motion sensors to deliver sham or active CLV (ClinicalTrials.gov identifier NCT04288245).
After 12 weeks of therapy composed of a miniaturized implant selectively activating the vagus nerve on successful movements, 19 people exhibited a significant beneficial effect on arm and hand strength and the ability to perform activities of daily living.
CLV represents a promising therapeutic avenue for people with chronic, incomplete cervical spinal cord injury.