Summary: In older mice, adult neurons in the primary visual cortex with an increased number of “silent synapses” and lacking PSD-95, showed the same structural changes only seen previously in younger animals.
Source: University of Göttingen
Understanding the cellular and molecular mechanisms underlying brain “plasticity”(how the brain can learn, develop and reorganise itself) is crucial for explaining many illnesses and conditions.
Neurocientists from the University of Göttingen and University Medical Center Göttingen (UMG) have now managed to repeatedly image synapses, the tiny contact sites between neurons, in awake adult mice. They are the first to discover that adult neurons in the primary visual cortex with an increased number of “silent synapses” (ie newly formed synapses that are inactivated), lacking a certain protein (PSD-95), display structural changes that were previously only reported in young mice.
This research by the Collaborative Research Centre CRC889 was published in PNAS.
It is well known that during early brain development, there are critical periods during which the brain is particularly plastic and individual experiences can trigger reorganization and adaptation in neuronal circuits. In developing brains, silent synapses are common and they help to functionally optimize the connections between principal neurons.
The research teams of Professor Siegrid Löwel (University of Göttingen) and Professor Oliver Schlüter (UMG) had already discovered that the maturation of silent synapses requires postsynaptic density protein-95 (PSD-95) and closes early critical periods. However, the specific processes that govern whether synaptic connections are kept or removed depending on experience were largely unknown.
To investigate this, the researchers imaged neurons from the mouse visual cortex with a two-photon microscope while the animal was awake before and after a particular visual experience.
As first author Rashad Yusifov from the University of Göttingen explains, “Previous studies have generally used anaesthetised mice but we now know that anesthesia itself can influence neuronal plasticity, which is why we did the current study in awake animals.”
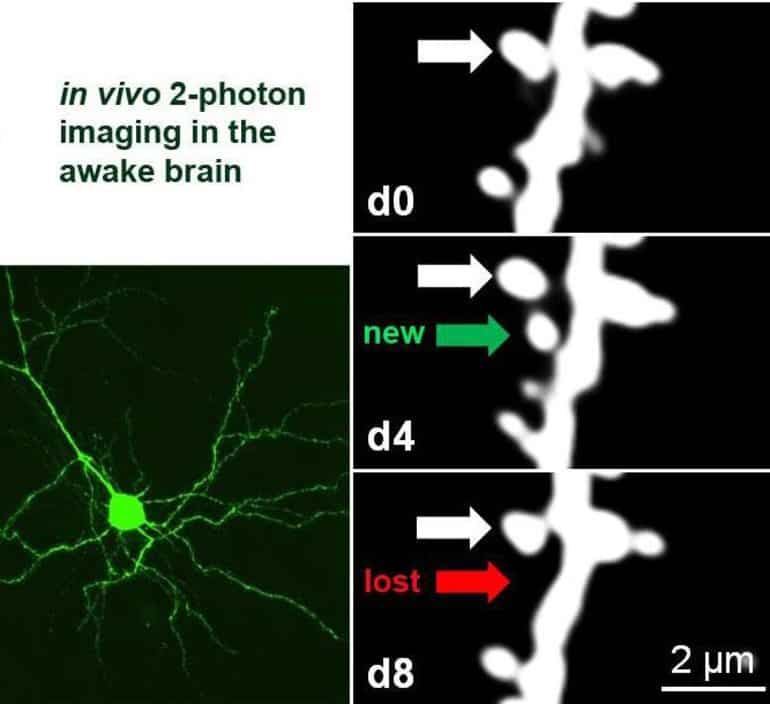
Yusifov goes on to say, “This challenging technique, which entails repeatedly locating and imaging very tiny structures – around one-thousandth of a millimetre – known as dendritic spines, can only be carried out in a few laboratories in the world”.
Researchers discovered that adult neurons deficient in PSD-95 display increased experience-dependent spine removal, an effect in the past only observed in young animals. Building on their previous discoveries, this collaborative research shows that PSD-95 deficient neurons display both functional and structural hallmarks of plasticity associated with a critical period. This means that neurons lacking PSD-95 keep a juvenile ability to restructure cortical connectivity even into adulthood.
Senior author Professor Löwel adds, “Answering these key questions will not only help to understand the rules underlying brain development, functioning and learning, but will also open up new avenues to develop clinically relevant concepts to promote regeneration and rehabilitation for diseased and injured brains.”
About this synaptic plasticity research news
Source: University of Göttingen
Contact: S Löwel – University of Göttingen
Image: The image is credited to S Löwel
Original Research: Open access.
“Spine dynamics of PSD-95-deficient neurons in the visual cortex link silent synapses to structural cortical plasticity” by S Löwel et al. PNAS
Abstract
Spine dynamics of PSD-95-deficient neurons in the visual cortex link silent synapses to structural cortical plasticity
Critical periods (CPs) are time windows of heightened brain plasticity during which experience refines synaptic connections to achieve mature functionality. At glutamatergic synapses on dendritic spines of principal cortical neurons, the maturation is largely governed by postsynaptic density protein-95 (PSD-95)-dependent synaptic incorporation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors into nascent AMPA-receptor silent synapses.
Consequently, in mouse primary visual cortex (V1), impaired silent synapse maturation in PSD-95-deficient neurons prevents the closure of the CP for juvenile ocular dominance plasticity (jODP). A structural hallmark of jODP is increased spine elimination, induced by brief monocular deprivation (MD).
However, it is unknown whether impaired silent synapse maturation facilitates spine elimination and also preserves juvenile structural plasticity. Using two-photon microscopy, we assessed spine dynamics in apical dendrites of layer 2/3 pyramidal neurons (PNs) in binocular V1 during ODP in awake adult mice. Under basal conditions, spine formation and elimination ratios were similar between PSD-95 knockout (KO) and wild-type (WT) mice.
However, a brief MD affected spine dynamics only in KO mice, where MD doubled spine elimination, primarily affecting newly formed spines, and caused a net reduction in spine density similar to what has been observed during jODP in WT mice. A similar increase in spine elimination after MD occurred if PSD-95 was knocked down in single PNs of layer 2/3.
Thus, structural plasticity is dictated cell autonomously by PSD-95 in vivo in awake mice. Loss of PSD-95 preserves hallmark features of spine dynamics in jODP into adulthood, revealing a functional link of PSD-95 for experience-dependent synapse maturation and stabilization during CPs.






