Summary: Awakening dormant stem cells could stimulate the growth of new neurons. The findings could have significant implications for the treatment of neurodegenerative diseases.
Source: Duke-NUS Medicial School
How dormant neural stem cells in fruit flies are activated and generate new neurons is described in a new research study by Duke-NUS Medical School. The findings could potentially help people with brain injury or neuronal loss if similar mechanisms apply in humans.
Publishing in PLOS Biology, the research team, led by Associate Professor Wang Hongyan, Deputy Director of Duke-NUS’ Neuroscience and Behavioural Disorders Programme and lead author of the study, described the process and molecules involved in reactivating fruit flies’ (also known by their scientific name, Drosophila) dormant neural stem cells, which can activate and generate new neurons. The ability of neural stem cells to switch from their dormant state and begin to proliferate is crucial in the brain. Until now, very little was known about how dormant neural stem cells become active.
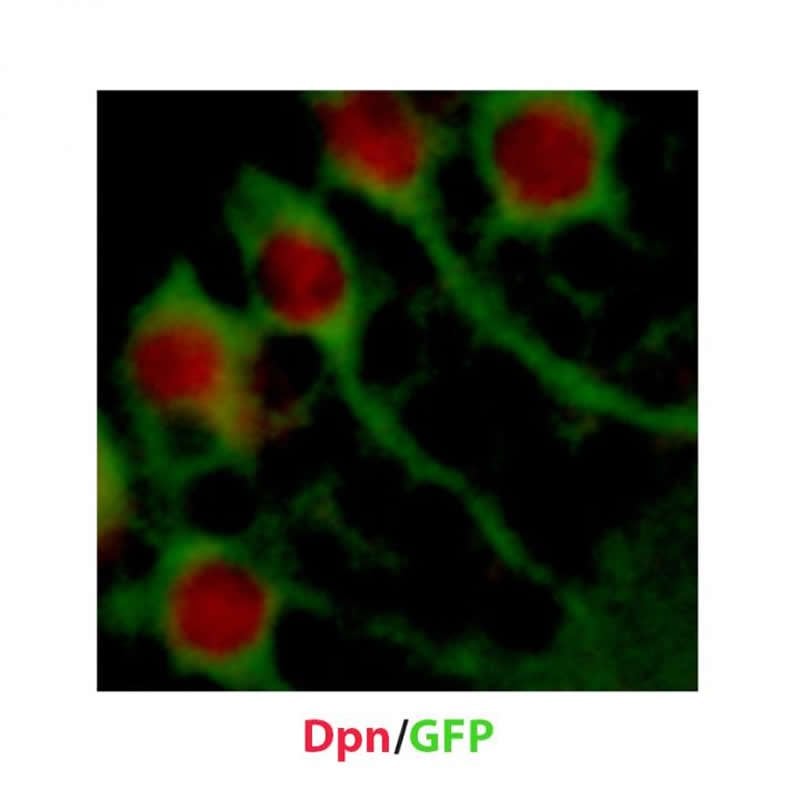
Assoc Prof Wang and colleagues investigated what factors are at play in developing Drosophila brains at the larval stage. They discovered that a protein complex called CRL4 is essential for the reactivation of neural stem cells as it downregulates a pathway that normally keeps neural stem cells in the dormant state. They saw that CRL4 forms a protein complex with the tumour suppressor Warts, a core component of the pathway, and that CRL4 targets Warts for degradation to trigger reactivation.
The ability to awaken dormant neural stem cells could stimulate new neurons to compensate for brain injury or the neuronal loss seen in neurodegenerative diseases, such as Parkinson’s or Alzheimer’s. Future work is required to confirm that CRL4 and the pathway it regulates works in a similar manner in mammalian brains.
“Mutations of human Cullin4B, a core component of the CRL4 complex, are associated with mental retardation and cortical malformations,” said Assoc Prof Wang. “Our work identifies the mechanisms behind CRL4 in fruit fly brain development and we plan to conduct further research to see if the same proteins are in play in mammals. Ultimately, our hope is that greater understanding and stimulation of these cells could eventually lead to therapeutic treatment of neurodevelopmental and neurodegenerative diseases.”
Professor Patrick Casey, Senior Vice Dean for Research at Duke-NUS, noted, “The prevalence of neurodegenerative diseases, such as Parkinson’s, is projected to increase in Singapore and worldwide in the coming decades, in tandem with increasingly ageing populations. Basic science research to better understand how the brain works, such as this study, is critical to developing new therapeutic strategies to enhance care for such diseases.”
Source:
Duke-NUS Medicial School
Media Contacts:
Federico Graciano – Duke-NUS Medicial School
Image Source:
The image is credited to Ye Sing Tan.
Original Research: Open access
“CRL4Mahj E3 ubiquitin ligase promotes neural stem cell reactivation”. Phuong Thao Ly, Ye Sing Tan, Chwee Tat Koe, Yingjie Zhang, Gengqiang Xie, Sharyn Endow, Wu-Min Deng, Fengwei Yu, Hongyan Wang.
PLOS Biology. doi:10.1371/journal.pbio.3000276
Abstract
CRL4Mahj E3 ubiquitin ligase promotes neural stem cell reactivation
The ability of neural stem cells (NSCs) to transit between quiescence and proliferation is crucial for brain development and homeostasis. Drosophila Hippo pathway maintains NSC quiescence, but its regulation during brain development remains unknown. Here, we show that CRL4Mahj, an evolutionarily conserved E3 ubiquitin ligase, is essential for NSC reactivation (exit from quiescence). We demonstrate that damaged DNA-binding protein 1 (DDB1) and Cullin4, two core components of Cullin4-RING ligase (CRL4), are intrinsically required for NSC reactivation. We have identified a substrate receptor of CRL4, Mahjong (Mahj), which is necessary and sufficient for NSC reactivation. Moreover, we show that CRL4Mahj forms a protein complex with Warts (Wts/large tumor suppressor [Lats]), a kinase of the Hippo signaling pathway, and Mahj promotes the ubiquitination of Wts. Our genetic analyses further support the conclusion that CRL4Mahj triggers NSC reactivation by inhibition of Wts. Given that Cullin4B mutations cause mental retardation and cerebral malformation, similar regulatory mechanisms may be applied to the human brain.