Summary: A new study reveals selenium is essential for the postnatal development of a specific type of interneuron.
Source: Helmholtz.
Exactly 200 years ago, the Swedish scientist Jöns Jacob Berzelius discovered the trace element selenium, which he named after the goddess of the moon, Selene. Besides its industrial applications (chemical industry, production of semiconductors and toners), selenium is an essential trace element and indispensable for humans, many animals and some bacteria. A team led by Dr. Marcus Conrad, research group leader at the Institute of Developmental Genetics (IDG) at Helmholtz Zentrum München, showed for the first time why selenium is a limiting factor for mammals.
Scientific ‘by-catch’ solves decades-old mystery
The scientists have been investigating for years the processes of a novel type of cell death, known as ferroptosis. In this context, the enzyme GPX4, which normally contains selenium in the form of the amino acid selenocysteine, plays an important role.
In order to better understand the role of GPX4 in this death process, we established and studied mouse models in which the enzyme was modified,” said study leader Conrad. “In one of these models, we observed that mice with a replacement of selenium to sulfur in GPX4 did not survive for longer than three weeks due to neurological complications.”
In their search for the underlying reasons, the researchers identified a distinct subpopulation of specialized neurons in the brain, which were absent when selenium-containing GPX4 was lacking. “In further studies, we were able to show that these neurons were lost during postnatal development, when sulfur- instead of selenium-containing GPX4 was present,” stated first author of the study, Irina Ingold.
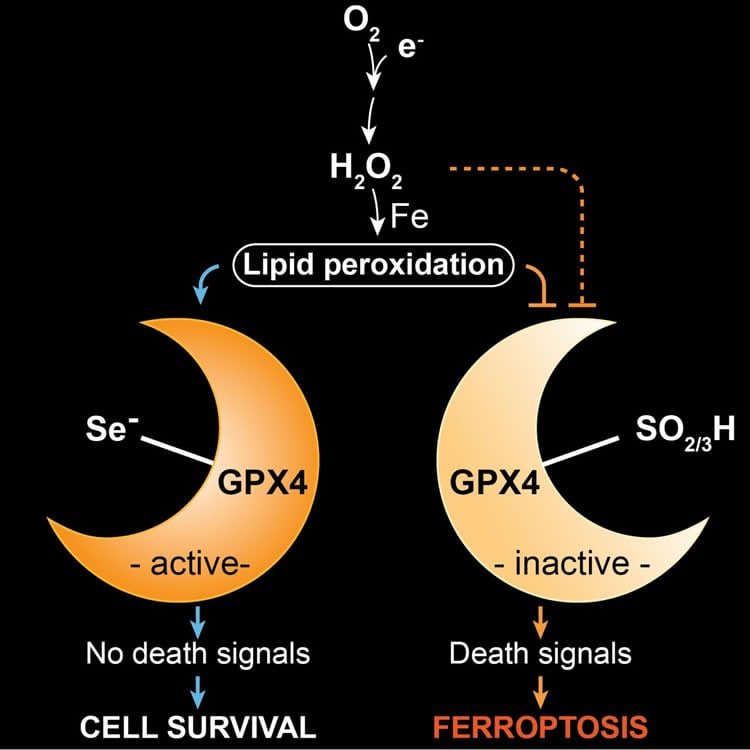
Furthermore, the scientists were able to show that ferroptosis is triggered by oxidative stress, which is known to occur for instance during high metabolic activity of cells and high neuronal activity. “Our study demonstrates for the first time that selenium is an essential factor for the postnatal development of a specific type of interneurons,” said Dr. José Pedro Friedmann Angeli, a scientist at the IDG, describing the results. “Selenium-containing GPX4 protects these specialized neurons from oxidative stress and from ferroptotic cell death.”
Thus, the study explains why certain selenoenzymes are essential in some organisms, including mammals, whereas they are dispensable in other organisms, such as fungi and higher plants. In future investigations, study leader Marcus Conrad and his team aim to investigate how ferroptosis is triggered in cells. As a long-term goal, he wants to elucidate the role of ferroptosis in various disease conditions in order to be able to alleviate diseases, such as cancer or neurodegeneration, which are currently difficult to tackle.
GPX4 stands for the enzyme glutathione peroxidase 4, one of 25 selenoproteins in humans. In the enzyme, selenium is an integral part of the 21st amino acid selenocysteine. The enzyme plays a decisive role in ferroptosis. The word ferroptosis, which means a type of programmed cell death dependent on iron, is derived from the Greek ptosis: fall and Latin ferrum: iron. Ferroptosis has not yet been completely elucidated, but the importance of cellular suicide has already been impressively confirmed, for example, by apoptosis, which has been more extensively studied.
Source: Marcus Conrad – Helmholtz
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Ingold et al., Cell.
Original Research: Abstract for “Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis” by Irina Ingold, Carsten Berndt, Sabine Schmitt, Sebastian Doll, Gereon Poschmann, Katalin Buday, Antonella Roveri, Xiaoxiao Peng, Florencio Porto Freitas, Tobias Seibt, Lisa Mehr, Michaela Aichler, Axel Walch, Daniel Lamp, Martin Jastroch, Sayuri Miyamoto, Wolfgang Wurst, Fulvio Ursini, Elias S.J. Arnér, Noelia Fradejas-Villar, Ulrich Schweizer, Hans Zischka, José Pedro Friedmann Angeli, and Marcus Conrad in Cell. Published online December 28 2017 doi:10.1016/j.cell.2017.11.048
[cbtabs][cbtab title=”MLA”]Helmholtz “Selenium Protects a Specific Type of Interneurons in the Brain.” NeuroscienceNews. NeuroscienceNews, 29 December 2017.
<https://neurosciencenews.com/selenium-interneurons-8242/>.[/cbtab][cbtab title=”APA”]Helmholtz (2017, December 29). Selenium Protects a Specific Type of Interneurons in the Brain. NeuroscienceNews. Retrieved December 29, 2017 from https://neurosciencenews.com/selenium-interneurons-8242/[/cbtab][cbtab title=”Chicago”]Helmholtz “Selenium Protects a Specific Type of Interneurons in the Brain.” https://neurosciencenews.com/selenium-interneurons-8242/ (accessed December 29, 2017).[/cbtab][/cbtabs]
Abstract
Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis
Highlights
•Selenium-containing GPX4 is necessary for full viability of mice
•The GPX4-Cys variant is highly susceptible to hydroperoxide-induced inactivation
•Hydroperoxide induces ferroptosis in Gpx4cys/cys cells
•GPX4-Cys bypasses the requirement of selenoproteins for cell viability
Summary
Selenoproteins are rare proteins among all kingdoms of life containing the 21st amino acid, selenocysteine. Selenocysteine resembles cysteine, differing only by the substitution of selenium for sulfur. Yet the actual advantage of selenolate- versus thiolate-based catalysis has remained enigmatic, as most of the known selenoproteins also exist as cysteine-containing homologs. Here, we demonstrate that selenolate-based catalysis of the essential mammalian selenoprotein GPX4 is unexpectedly dispensable for normal embryogenesis. Yet the survival of a specific type of interneurons emerges to exclusively depend on selenocysteine-containing GPX4, thereby preventing fatal epileptic seizures. Mechanistically, selenocysteine utilization by GPX4 confers exquisite resistance to irreversible overoxidation as cells expressing a cysteine variant are highly sensitive toward peroxide-induced ferroptosis. Remarkably, concomitant deletion of all selenoproteins in Gpx4cys/cys cells revealed that selenoproteins are dispensable for cell viability provided partial GPX4 activity is retained. Conclusively, 200 years after its discovery, a specific and indispensable role for selenium is provided.
“Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis” by Irina Ingold, Carsten Berndt, Sabine Schmitt, Sebastian Doll, Gereon Poschmann, Katalin Buday, Antonella Roveri, Xiaoxiao Peng, Florencio Porto Freitas, Tobias Seibt, Lisa Mehr, Michaela Aichler, Axel Walch, Daniel Lamp, Martin Jastroch, Sayuri Miyamoto, Wolfgang Wurst, Fulvio Ursini, Elias S.J. Arnér, Noelia Fradejas-Villar, Ulrich Schweizer, Hans Zischka, José Pedro Friedmann Angeli, and Marcus Conrad in Cell. Published online December 28 2017 doi:10.1016/j.cell.2017.11.048







