During the short, dreary days of winter, do you feel tired and oversleep, tend to be depressed and irritable and have trouble concentrating, but once spring comes you feel just fine?
If so, you may be one of the 4 to 6 percent of the American public who suffer from seasonal affective disorder (SAD), a type of depression that occurs during the same season each year.
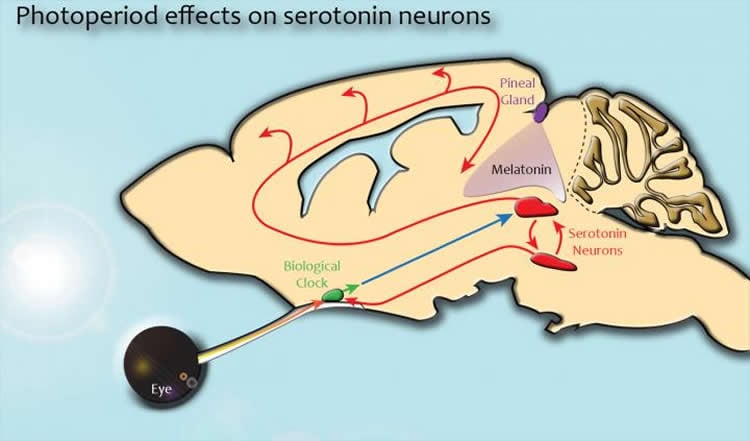
Biologists have known that variations in the amount of sunlight a person receives and her or his circadian clock play a role in the disorder. They have also proposed that the neurotransmitters serotonin and melatonin may be involved. However, they have not yet identified the underlying neurobiological mechanisms responsible.
Now, a team of Vanderbilt biologists has taken a major step toward this goal. In the May 7 issue of the journal Current Biology, they report that they have localized the seasonal light cycle effects that drive SAD to a small region in the mid-brain called the dorsal raphe nucleus in an experiment with mice, a common “animal model” for studying depression in humans.
In both mice and humans, the dorsal raphe nucleus is an area where many of the specialized neurons that control serotonin levels throughout the brain are located. Because high concentrations of serotonin are associated with feelings of well-being and happiness while low levels are linked to depression, it plays a major role in regulating an individual’s mood. Their study also found that the day/night cycle in which individuals are born can have a long-lasting effect on the activity level of the neurons in this region.
“We got the idea for the study from a report by Viennese psychiatrists which found a season of birth correlation in SAD patients,” said the study’s lead author, graduate student Noah Green.
The biologists decided to concentrate their investigation on the dorsal raphe nucleus because prior studies had shown that it is linked to the brain’s master biological clock and it also responds to melatonin, a hormone that is involved in the regulation of a number of related physiological functions, including sleep, blood pressure and seasonal reproduction.
To test the effect of seasonal light cycles, they divided mice into three groups. One group was born and raised in an environment with a summer-like light cycle of 16 hours of light and eight hours of dark. The second group was born and raised with a cycle of 12 hours of light and 12 hours of dark, like spring and fall. The third group was born and raised in a winter-like light cycle with eight hours of light and 16 hours of dark. Other than the light cycle the environments were identical.
Scientists have developed a number of tests to determine whether mice are depressed or, more precisely, exhibit depression-like behavior. For example, one of these is a forced swim test, where the researchers put a mouse in a pool of water and measure how much time it spends struggling to get out and how much time it spends floating passively. They argue that a depressed mouse will spend less time struggling than a normal mouse. (Mice float so they aren’t harmed when they stop paddling.)
Administering several of these tests, the Vanderbilt researchers found that the summer-light-cycle mice exhibited lower levels of depression-like behavior than their spring/fall- or winter-light-cycle counterparts.
When the biologists examined the brains of individuals from the three groups, they found a story consistent with the behavioral testing. They found that serotonergic neurons fire faster in the summer-light-cycle mice and they had elevated levels of serotonin and the neurotransmitter norepinephrine, which is known to excite serotonergic neurons.
“Before, we thought serotonin was probably involved. Now we know that serotonergic neurons are definitely involved,” said Vanderbilt’s Stevenson Chair in Biological Sciences Douglas McMahon, who supervised the study.
Intriguingly, when mice born in summer light cycles were switched to winter light cycles the increased firing of serotonin neurons persisted for several months, into adulthood for the mice.
“This showed that early life seasonal photoperiods can have enduring effects on the serotonin neurons. If such an effect occurs in humans, and is long-lasting, it could contribute to the season of birth modulation of SAD risk,” said McMahon.
The researchers also had an opportunity to test the role of melatonin. Normally, the hormone affects the serotonergic neurons in the raphe nucleus by binding to a special structure on the neurons’ surface called melatonin receptor 1.
So the researchers got a strain of genetically engineered mice with melatonin receptor 1 “knocked out.” Although the pineal glands in these mice worked normally, their serotonergic neurons couldn’t respond to the melatonin because they lacked the proper receptors.
When they raised the knock-out mice in the three different photoperiods, the researchers found that the seasonal effect disappeared.
Vanderbilt post-doctoral fellows Chad Jackson and Hideki Iwamoto and doctoral student Michael Tackenberg also contributed to the study.
Funding: This research was funded by the National Institutes of Health grants R01 EY015815 and P50 MN096972 and a pilot grant from the Vanderbilt Silvio S. Conte Center.
Source: David Salisbury – Vanderbilt University
Image Credit: The image is credited to Chris Ciarleglio, Brown University
Original Research: Abstract for “Photoperiod Programs Dorsal Raphe Serotonergic Neurons and Affective Behaviors” by Noah H. Green, Chad R. Jackson, Hideki Iwamoto, Michael C. Tackenberg, and Douglas G. McMahon in Current Biology. Published online May 7 2015 doi:10.1016/j.cub.2015.03.050
Abstract
Photoperiod Programs Dorsal Raphe Serotonergic Neurons and Affective Behaviors
Highlights
•Summer-like long photoperiods increase serotonin neuron excitability and firing
•Long photoperiods increase serotonin and norepinephrine levels in the midbrain
•Long photoperiods during development induce lasting increases in firing rate
•Knockout of the MT1 receptor negates the observed photoperiodic changes
Summary
The serotonergic raphe nuclei of the midbrain are principal centers from which serotonin neurons project to innervate cortical and sub-cortical structures. The dorsal raphe nuclei receive light input from the circadian visual system [ 1 ] and indirect input from the biological clock nuclei [ 2, 3 ]. Dysregulation of serotonin neurotransmission is implicated in neurobehavioral disorders, such as depression and anxiety [ 4 ], and alterations in the serotonergic phenotype of raphe neurons have dramatic effects on affective behaviors in rodents [ 5 ]. Here, we demonstrate that day length (photoperiod) during development induces enduring changes in mouse dorsal raphe serotonin neurons—programming their firing rate, responsiveness to noradrenergic stimulation, intrinsic electrical properties, serotonin and norepinephrine content in the midbrain, and depression/anxiety-related behavior in a melatonin receptor 1 (MT1)-dependent manner. Our results establish mechanisms by which seasonal photoperiods may dramatically and persistently alter the function of serotonin neurons.
“Photoperiod Programs Dorsal Raphe Serotonergic Neurons and Affective Behaviors” by Noah H. Green, Chad R. Jackson, Hideki Iwamoto, Michael C. Tackenberg, and Douglas G. McMahon in Current Biology. Published online May 7 2015 doi:10.1016/j.cub.2015.03.050






