Summary: According to a new study, nicotine reduces dorsal striatal output, underlying the urge to smoke and making it difficult to quite the addiction.
Source: SfN.
A study of rat brain slices published in Journal of Neuroscience demonstrates how nicotine interacts with cells that regulate the output of a brain region involved in habit formation. The research could inform efforts to help people quit smoking and avoid relapse.
The addictive qualities of nicotine have been attributed to the brain’s reward system. However, recent research suggests that a shift of activity from the ventral to the dorsal striatum, which parallels the transition of an intentional behavior into a more automatic habit, may have an important role in the development of nicotine addiction.
Louise Adermark and colleagues found that nicotine reduces dorsal striatal output, an effect that persists even after the drug has been cleared from the brain. These changes in neuronal activity may underlie the urge to smoke as well as make it difficult to break the habit. This advance in our understanding of nicotine addiction may help to decrease smoking prevalence.
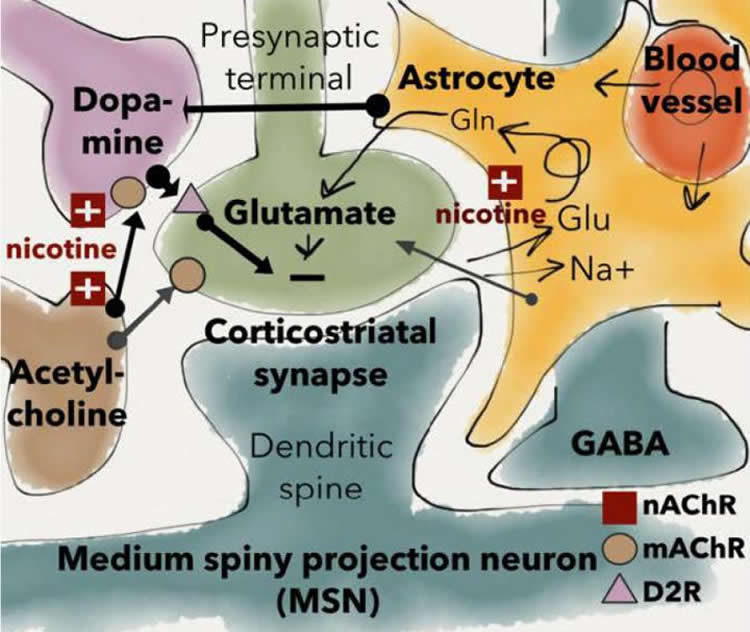
Funding: Stiftelsen Psykiatriska Forskningsfonden, Swedish Brain Foundation, Swedish Medical Research Council funded this study.
Source: David Barnstone – SfN
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Licheri et al., JNeurosci (2018).
Original Research: Abstract for “Complex control of striatal neurotransmission by nicotinic acetylcholine receptors via excitatory inputs onto medium spiny neurons” by Valentina Licheri, Oona Lagström, Amir Lotfi, Mary H. Patton, Holger Wigström, Brian Mathur and Louise Adermark in Journal of Neuroscience. Published June 25 2018.
doi:10.1523/JNEUROSCI.0071-18.2018
[cbtabs][cbtab title=”MLA”]SfN “Nicotine Alters Neurotransmission in Habit Forming Brain Region.” NeuroscienceNews. NeuroscienceNews, 25 June 2018.
<https://neurosciencenews.com/nicotine-striatum-9441/>.[/cbtab][cbtab title=”APA”]SfN (2018, June 25). Nicotine Alters Neurotransmission in Habit Forming Brain Region. NeuroscienceNews. Retrieved June 25, 2018 from https://neurosciencenews.com/nicotine-striatum-9441/[/cbtab][cbtab title=”Chicago”]SfN “Nicotine Alters Neurotransmission in Habit Forming Brain Region.” https://neurosciencenews.com/nicotine-striatum-9441/ (accessed June 25, 2018).[/cbtab][/cbtabs]
Abstract
Complex control of striatal neurotransmission by nicotinic acetylcholine receptors via excitatory inputs onto medium spiny neurons
The prevalence of nicotine dependence is higher than that for any other substance abuse disorder, still the underlying mechanisms are not fully established. To this end, we studied acute effects by nicotine on neurotransmission in the dorsolateral striatum (DLS), a key brain region with respect to the formation of habits. Electrophysiological recordings in acutely isolated brain slices from rodent showed that nicotine (10 nM-10 μM) produced a long-term depression (LTD) of evoked field potentials. Current clamp recordings revealed no significant effect by nicotine on membrane voltage or action potential frequency, indicating that the effect by nicotine is primarily synaptic. Nicotine did not modulate spontaneous inhibitory postsynaptic currents (sIPSCs), or the connectivity between fast-spiking interneurons and medium spiny neurons, as assessed by whole cell recordings combined with optogenetics. However, the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) was significantly depressed by nicotine. The effect by nicotine was mimicked by agonists targeting α7- or α4-containing nicotinic acetylcholine receptors (nAChRs), and blocked in slices pre-treated with a cocktail of antagonists targeting these receptor subtypes. Nicotine-induced LTD was furthermore inhibited by dopamine D2 receptor antagonist and occluded by D2 receptor agonist. In addition, modulation of cholinergic neurotransmission suppressed the responding to nicotine, which might reflect upon the postulated role for nAChRs as a presynaptic filter to differentially govern dopamine release depending on neuronal activity. Nicotine-induced suppression of excitatory inputs onto medium spiny neurons may promote nicotine-induced locomotor stimulation, and putatively initiate neuroadaptations that could contribute to the transition towards compulsive drug taking.
SIGNIFICANCE STATEMENT
In order to decrease smoking prevalence factors that may contribute to the development of nicotine addiction needs to be identified. The data presented here shows that nicotine suppresses striatal neurotransmission by selectively reducing the frequency of excitatory inputs to medium spiny neurons (MSNs), while rendering excitability, inhibitory neurotransmission and FSI-MSN connectivity unaltered. In addition, we show that the effect displayed by nicotine outlasts the presence of the drug, which could be fundamental for the addictive properties of nicotine. Considering the inhibitory tone displayed by MSNs on dopaminergic cell bodies and local terminals, nicotine-induced long-lasting depression of striatal output could play a role in behavioral transformations associated with nicotine use, and putatively elicit neuroadaptations underlying compulsive drug-seeking habits.