Summary: A new study reports hippocampal neural stem cells regulate their own cell fate via the protein Drosha.
Source: University of Basel.
To date, it has been assumed that the differentiation of stem cells depends on the environment they are embedded in. A research group at the University of Basel now describes for the first time a mechanism by which hippocampal neural stem cells regulate their own cell fate via the protein Drosha. The journal Cell Stem Cell has published their results.
Stem cells are undifferentiated cells that have the potential to differentiate into many cell types. However, the cell types that somatic stem cells produce are usually restricted to those of the organ in which they sit. The current view proposes that stem cell differentiation is controlled by their local environment, the so-called niche. Thus, stem cells receive and interpret specific factors present in their niche that guide their differentiation into specific and restricted cell types.
In the adult brain, the hippocampus is responsible for specific forms of memory – a brain region that is also affected in diseases such as dementia, depression and epilepsy. The functions of the hippocampus are based on different cell types, some of which are generated throughout life by neural stem cells. Neural stem cells are generally accepted to produce three different cell types: neurons, astrocytes and oligodendrocytes. However, the adult hippocampus does not produce oligodendrocytes – the reason for this was so far not known.
Intrinsic cell mechanism
Researchers from the Department of Biomedicine at the University of Basel have now found that the fate of adult hippocampal stem cells is not only controlled by their local niche, but also by a cell-intrinsic mechanism. Their study describes the central role of the enzyme Drosha in this mechanism. Drosha degrades the messenger RNA for NFIB in the adult hippocampal stem cells and prevents the expression of this transcription factor which is necessary for the differentiation of oligodendrocytes and thus blocks their development and therefore biases differentiation towards neurons.
The team lead by Prof. Verdon Taylor was able to demonstrate for the first time a cell-intrinsic mechanism regulating stem cell fate. «Our research results about the function of Drosha challenge the way we used to think about how stem cell fate is controlled», says cell biologist Taylor. His research group now wants to study if and how stem cells are able to modulate the activity of Drosha in order to satisfy demand.
Source: Olivia Poisson – University of Basel
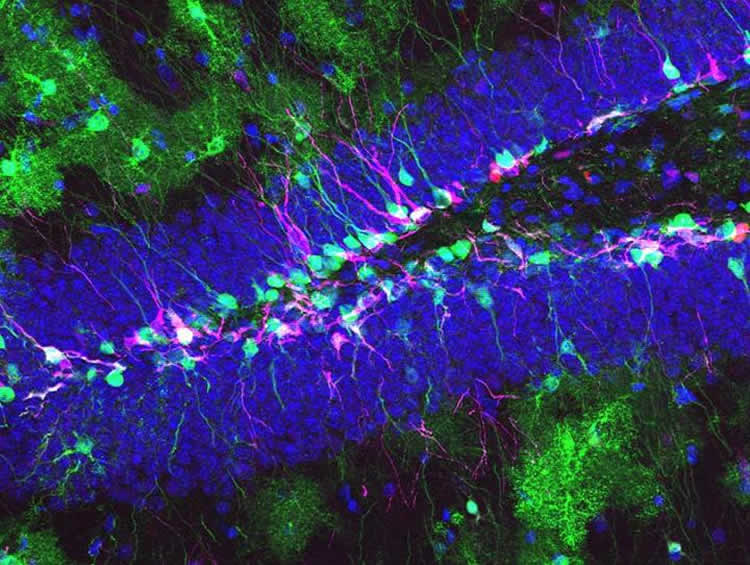
Image Source: This NeuroscienceNews.com image is credited to Department of Biomedicine, University of Basel.
Original Research: Abstract for “Multipotency of Adult Hippocampal NSCs In Vivo Is Restricted by Drosha/NFIB” by Chiara Rolando, Andrea Erni, Alice Grison, Robert Beattie, Anna Engler, Paul J. Gokhale, Marta Milo, Thomas Wegleiter, Sebastian Jessberger, and Verdon Taylor in Cell Stem Cell. Published online August 18 2016 doi:10.1016/j.stem.2016.07.003
[cbtabs][cbtab title=”MLA”]University of Basel. “Neural Stem Cells Control Their Own Fate.” NeuroscienceNews. NeuroscienceNews, 18 August 2016.
<https://neurosciencenews.com/neural-stem-cells-genetics-4869/>.[/cbtab][cbtab title=”APA”]University of Basel. (2016, August 18). Neural Stem Cells Control Their Own Fate. NeuroscienceNews. Retrieved August 18, 2016 from https://neurosciencenews.com/neural-stem-cells-genetics-4869/[/cbtab][cbtab title=”Chicago”]University of Basel. “Neural Stem Cells Control Their Own Fate.” https://neurosciencenews.com/neural-stem-cells-genetics-4869/ (accessed August 18, 2016).[/cbtab][/cbtabs]
Abstract
Multipotency of Adult Hippocampal NSCs In Vivo Is Restricted by Drosha/NFIB
Highlights
•Drosha regulates adult hippocampal stem cell maintenance
•Drosha inhibits oligodendrocytic differentiation of adult stem cells
•Drosha targets NFIB mRNA hairpin to inhibit expression and enable neurogenesis
•NFIB expression induces oligodendrocytic fate in adult hippocampal stem cells
Summary
Adult neural stem cells (NSCs) are defined by their inherent capacity to self-renew and give rise to neurons, astrocytes, and oligodendrocytes. In vivo, however, hippocampal NSCs do not generate oligodendrocytes for reasons that have remained enigmatic. Here, we report that deletion of Drosha in adult dentate gyrus NSCs activates oligodendrogenesis and reduces neurogenesis at the expense of gliogenesis. We further find that Drosha directly targets NFIB to repress its expression independently of Dicer and microRNAs. Knockdown of NFIB in Drosha-deficient hippocampal NSCs restores neurogenesis, suggesting that the Drosha/NFIB mechanism robustly prevents oligodendrocyte fate acquisition in vivo. Taken together, our findings establish that adult hippocampal NSCs inherently possess multilineage potential but that Drosha functions as a molecular barrier preventing oligodendrogenesis.
“Multipotency of Adult Hippocampal NSCs In Vivo Is Restricted by Drosha/NFIB” by Chiara Rolando, Andrea Erni, Alice Grison, Robert Beattie, Anna Engler, Paul J. Gokhale, Marta Milo, Thomas Wegleiter, Sebastian Jessberger, and Verdon Taylor in Cell Stem Cell. Published online August 18 2016 doi:10.1016/j.stem.2016.07.003