Summary: Measles virus that persists in the body can develop mutations in the F protein, which controls how the virus infects cells. The mutated protein can interact with its normal form, making it capable of infecting the brain.
Source: Kyushu University
Researchers in Japan have uncovered the mechanism for how the measles virus can cause subacute sclerosing panencephalitis, or SSPE, a rare but fatal neurological disorder that can occur several years after a measles infection.
Although the normal form of the measles virus cannot infect the nervous system, the team found that viruses that persist in the body can develop mutations in a key protein that controls how they infect cells. The mutated proteins can interact with its normal form, making it capable of infecting the brain.
Their findings were reported in the journal Science Advances.
If you are of a certain age, you may have gotten the measles as a child. Many born after the 1970s have never gotten it thanks to vaccines. The condition is caused by the virus of the same name, which is one of the most contagious pathogens to this day. The World Health Organization estimates that nearly nine million people worldwide were infected with measles in 2021, with the number of deaths reaching 128,000.
“Despite its availability, the recent COVID-19 pandemic has set back vaccinations, especially in the Global South,” explains Yuta Shirogane, Assistant Professor at Kyushu University’s Faculty of Medical Sciences. “SSPE is a rare but fatal condition caused by the measles virus. However, the normal measles virus does not have the ability to propagate in the brain, and thus it is unclear how it causes encephalitis.”
A virus infects cells through a series of proteins that protrude from its surface. Usually, one protein will first facilitate the virus to attach to a cell’s surface, then another surface protein will cause a reaction that lets the virus into the cell, leading to an infection. Therefore, what a virus can or cannot infect can depend heavily on the type of cell.
“Usually, the measles virus only infects your immune and epithelial cells, causing the fever and rash,” continues Shirogane. “Therefore, in patients with SSPE, the measles virus must have remained in their body and mutated, then gained the ability to infect nerve cells. RNA viruses like measles mutate and evolve at very high rates, but the mechanism of how it evolved to infect neurons has been a mystery.”
The key player in allowing the measles virus to infect a cell is a protein called fusion protein, or F protein. In the team’s previous studies, they showed that certain mutations in the F protein puts it in a ‘hyperfusongenic’ state, allowing it to fuse onto neural synapses and infect the brain.
In their latest study, the team analyzed the genome of the measles virus from SSPE patients and found that various mutations had accumulated in their F protein. Interestingly, certain mutations would increase infection activity while others actually decreased it.
“This was surprising to see, but we found an explanation. When the virus infects a neuron, it infects it through ‘en bloc transmission,’ where multiple copies of the viral genome enter the cell,” continues Shirogane. “In this case, the genome encoding the mutant F protein is transmitted simultaneously with the genome of the normal F protein, and both proteins are likely to coexist in the infected cell.”
Based on this hypothesis, the team analyzed the fusion activity of mutant F proteins when normal F proteins were present. Their results showed that fusion activity of a mutant F protein is suppressed due to interference from the normal F proteins, but that interference is overcome by the accumulation of mutations in the F protein.
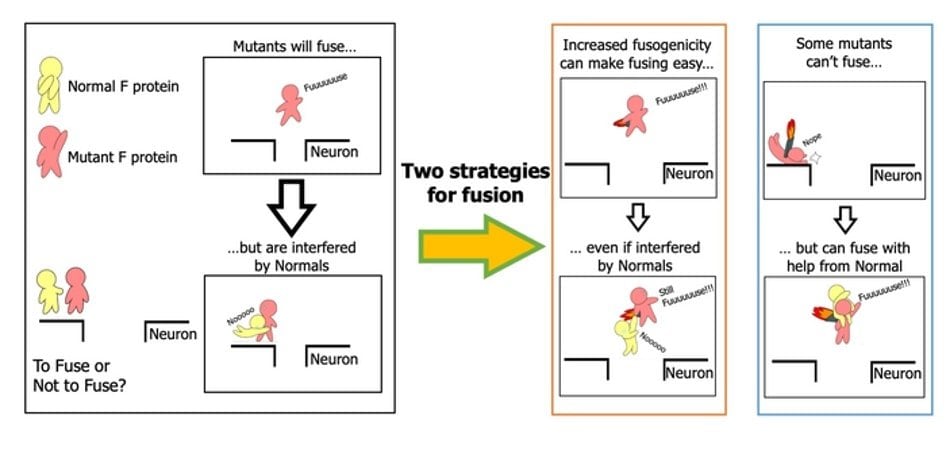
In another case, the team found that a different set of mutations in the F protein results in a completely opposite result: a reduction in fusion activity. However, to their surprise, this mutation can actually cooperate with normal F proteins to increase fusion activity. Thus, even mutant F proteins that appear to be unable to infect neurons can still infect the brain.
“It is almost counter to the ‘survival of the fittest’ model for viral propagation. In fact, this phenomenon where mutations interfere and/or cooperate with each other is called ‘Sociovirology.’ It’s still a new concept, but viruses have been observed to interact with each other like a group. It’s an exciting prospect” explains Shirogane.
The team hopes that their results will help develop therapeutics for SSPE, as well as elucidate the evolutionary mechanisms common to viruses that have similar infection mechanisms to measles such as novel coronaviruses and herpesviruses.
“There are many mysteries in the mechanisms by which viruses cause diseases. Since I was a medical student, I was interested in how the measles virus caused SSPE. I am happy that we were able to elucidate the mechanism of this disease,” concludes Shirogane.
About this neurology and virology research news
Author: Raymond Terhune
Source: Kyushu University
Contact: Raymond Terhune – Kyushu University
Image: The image is credited to Kyushu University/Hidetaka Harada/Yuta Shirogane
Original Research: Open access.
“Collective Fusion Activity Determines Neurotropism of an en Bloc Transmitted Enveloped Virus” by Yuta Shirogane et al. Science Advances
Abstract
Collective Fusion Activity Determines Neurotropism of an en Bloc Transmitted Enveloped Virus
Measles virus (MeV), which is usually non-neurotropic, sometimes persists in the brain and causes subacute sclerosing panencephalitis (SSPE) several years after acute infection, serving as a model for persistent viral infections.
The persisting MeVs have hyperfusogenic mutant fusion (F) proteins that likely enable cell-cell fusion at synapses and “en bloc transmission” between neurons.
We here show that during persistence, F protein fusogenicity is generally enhanced by cumulative mutations, yet mutations paradoxically reducing the fusogenicity may be selected alongside the wild-type (non-neurotropic) MeV genome.
A mutant F protein having SSPE-derived substitutions exhibits lower fusogenicity than the hyperfusogenic F protein containing some of those substitutions, but by the wild-type F protein coexpression, the fusogenicity of the former F protein is enhanced, while that of the latter is nearly abolished.
These findings advance the understanding of the long-term process of MeV neuropathogenicity and provide critical insight into the genotype-phenotype relationships of en bloc transmitted viruses.






