Summary: A breakthrough tool called Helicase-Assisted Continuous Editing (HACE) allows scientists to create precise genetic mutations in specific genes without affecting the rest of the genome. By combining helicase enzymes with CRISPR technology, HACE introduces mutations into targeted DNA sequences, advancing our ability to study gene functions and disease mechanisms. The tool has already identified drug resistance mutations in cancer-related genes and splicing defects in blood cancers, showcasing its potential for therapeutic discovery.
Key Facts:
- HACE introduces mutations in specific genes, leaving the rest of the genome untouched.
- It has pinpointed mutations linked to cancer drug resistance and splicing defects.
- This tool could revolutionize therapeutic discovery and genomic research.
Source: Harvard
Gene mutations have consequences both good and bad — from resistance to conditions like diabetes to susceptibility to certain cancers.
In order to study these mutations, scientists need to introduce them directly into human cells. But changing genetic instructions inside cells is complex. The human genome comprises 3 billion base pairs of DNA divided across tens of thousands of genes.
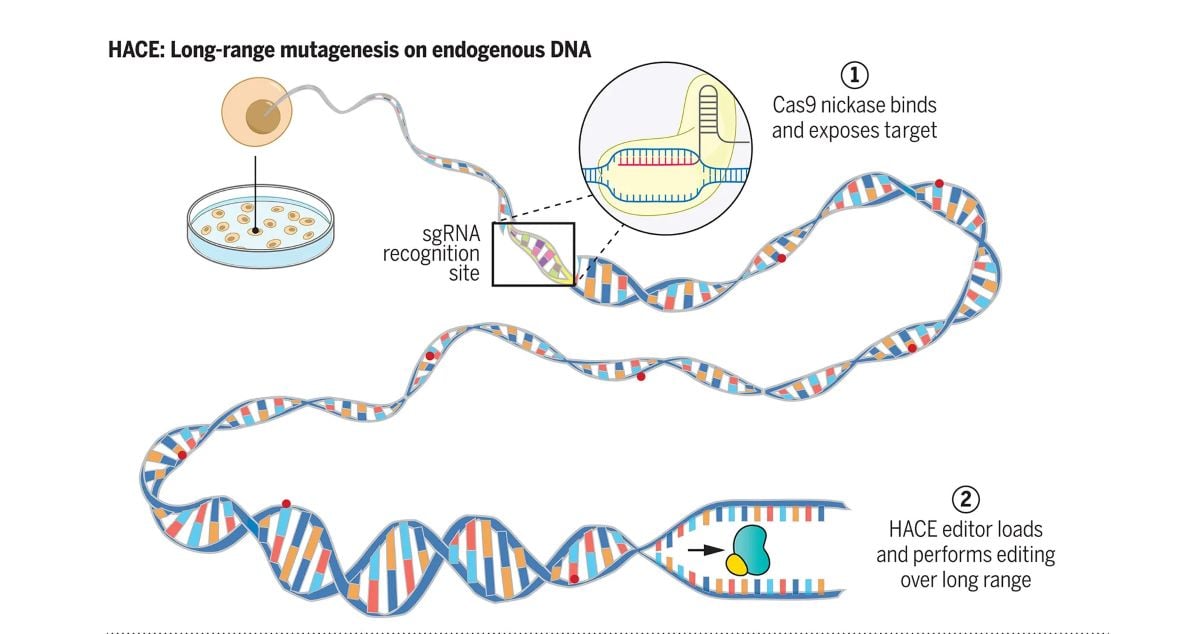
To that end, Harvard researchers have created a tool that allows them to rapidly create mutations only in particular genes of interest without disturbing the rest of the genome. Described in Science, their tool, called Helicase-Assisted Continuous Editing (HACE), can be deployed to predetermined regions of the genome in intact, living cells.
“The development of tools like this marks a significant leap forward in our ability to harness evolution directly within human cells,” said first author Xi Dawn Chen, a Griffin Graduate School of Arts and Sciences student studying synthetic biology in the Department of Stem Cell and Regenerative Biology.
“By allowing targeted mutagenesis in specific parts of the genome, this tool opens the door to creating enzymes and treatments that were previously out of reach.”
Unlike current methods for mutagenesis, which involve inserting extra copies of genes or broadly mutating many different genes at once, HACE offers the advantage of being directed to locations — like going to a specific address, rather than a neighborhood. The team’s novel bioengineering involves combining a helicase, which is an enzyme that naturally “unzips” DNA, with a gene-editing enzyme.
They then use the gene-editing technology CRISPR-Cas9 to guide the protein pair to the gene they want to mutate. As the helicase unzips the DNA, it introduces mutations into only that gene sequence.
“HACE combines CRISPR’s precision with the ability to edit long stretches of DNA, making it a powerful tool for targeted evolution,” explained senior author Fei Chen, assistant professor in the Department of Stem Cell and Regenerative Biology and member at the Broad Institute.
To demonstrate the tool’s power in the lab, the scientists used it to identify drug resistance mutations in a gene called MEK1, which cancer treatments often target but frequently fail because the diseased cells mutate resistance mechanisms.
Using HACE, the team sequenced only those mutated genes and pinpointed several unique changes associated with resistance to cancer drugs like trametinib and selumetinib, offering insights into how mutations affect drug performance.
They also examined how mutations in SF3B1, a gene involved in a biomolecular process called RNA splicing, affects RNA assembly. Mutations in this gene are common in blood cancers, but it’s been unclear which mutations cause the splicing defects; with HACE, the team could easily identify those changes.
And in partnership with Bradley Bernstein’s lab at Harvard Medical School and Dana-Farber Cancer Institute, the researchers also used the tool to better understand how changes in a regulatory DNA region affect the production of a protein in immune cells recognized as a potential target for cancer immunotherapies.
Bernstein said tools like HACE could someday allow massive edits of gene regulatory sequences that could then be coupled with deep learning computation for deciphering. “One can imagine many new therapeutic opportunities that involve precise edits or tuning of these regulatory sequences to ‘fix’ gene activity and ameliorate disease,” Bernstein said.
Funding: This research was supported by multiple sources including the National Institutes of Health, the Broad Institute, and the Harvard Stem Cell Institute.
About this genetics research news
Author: Anne J. Manning
Source: Harvard
Contact: Anne J. Manning – Harvard
Image: The image is credited to Neuroscience News
Original Research: Closed access.
“Helicase-assisted continuous editing for programmable mutagenesis of endogenous genomes” by Xi Dawn Chen et al. Science
Abstract
Helicase-assisted continuous editing for programmable mutagenesis of endogenous genomes
INTRODUCTION
A fundamental challenge of genomics is to chart the impact of the three billion bases in the human genome on protein function and gene regulation. Thus, a critical goal is to develop strategies for mutagenizing genomic sequences systematically and at high throughput.
In particular, targeted mutagenesis of single genomic loci could emulate the natural evolution process to reveal sequence-structure relationships, gain- and loss-of-function phenotypes, and cooperative mutations. However, no method exists that can perform continuous mutagenesis at targeted regions in the endogenous genomes of mammalian cells.
RATIONALE
We sought to develop a tool to perform targeted mutagenesis on the endogenous mammalian genome. Looking to nature, we observed that helicases are highly processive enzymes that can traverse large genomic regions. Some helicases, including those involved in DNA damage repair, can load and start unwinding DNA at single-stranded DNA regions in the genome.
We reasoned that such helicases could be used for long-range targeted mutagenesis when fused to a deaminase enzyme. The fusion construct and its interval of hypermutation could then be programmably targeted, through single-guide RNAs (sgRNAs), to specific genomic regions using a Cas9 nickase. The directional and long-range DNA-unwinding event by the recruited helicase will then generate random mutations in the region.
RESULTS
We designed a platform called helicase-assisted continuous editing (HACE), which combines long-range editing of entire loci with the sequence programmability inherent to CRISPR gene editing tools. HACE uses CRISPR-Cas9 to direct the loading of a helicase-deaminase fusion for targeted hypermutation of the downstream genomic sequence. HACE achieved locus-specific deamination across >1000 nucleotides with mutations continuously accumulating over time.
We further evaluated HACE prototypes incorporating diverse helicases, Cas9 variants, and deaminases, showing that they have tunable edit rates and ranges. We also showed that HACE can be multiplexed to target multiple genomic regions with a minimal number of guide RNAs. We then applied HACE in coding and noncoding genomic contexts to functionally dissect endogenous mutations conferring drug resistance, changes in enzymatic activity, and altered cis-regulatory element function.
In the coding space, we identified variants that lead to mitogen-activated protein kinase kinase 1 (MEK1)–inhibitor drug resistance and also identified variants in SF3B1, a splicing factor, that lead to alternative 3′ splice-site usage. Turning to regulatory regions, we defined functional artificial variants in the enhancer regions of CD69 and pinpointed specific bases and motifs that mediate the impact of RUNX transcription factors on CD69 regulation.
HACE solves two limitations faced by conventional base editing screens: the requirement of an NGG protospacer adjacent motif in the sgRNA recognition sequence and the occurrence of bystander mutations that can create artificial linkages and confound screening results. The long editing range of HACE can also uncover combinatorial effects and interactions between multiple distant mutations across a locus.
CONCLUSION
HACE makes possible the continuous, long-range, programmable diversification of endogenous mammalian genomes. We envision that HACE will substantially expand the functional genomics toolbox and enable the building of systematic sequence-function maps of both coding and noncoding genomes.
Furthermore, HACE can be developed into a directed evolution system in the endogenous genome, enabling the selection of sequences for desired functions in mammalian biology.