Summary: Cellular reprogramming of glioma cells into neural cells may be a promising treatment for glioblastoma brain cancer.
Source: Cactus Communications
Gliomas are the most common primary brain tumors in adults. Among them, the high-grade glioblastomas (GBMs) are particularly known to be notoriously aggressive and invasive, which makes it challenging to treat them. The disease rapidly progresses to an advanced stage in a short span of time, and the survival of such patients is often dismal.
Despite substantial research, targeted therapies that can improve the survival rate and quality of life of patients with GBM are lacking. Current therapies are focused on inhibiting tumor growth or destroying the cancer cells. However, given the high adaptability, resistance, and diversity of these tumors, such therapies alone are insufficient.
GBMs are caused by the uncontrolled division of glial cells that nourish the neurons and make up the supportive matrix of the brain tissue. Unlike neurons, which do not divide, glial cells can undergo cell division, making them susceptible to tumor formation. Taking advantage of this distinction between the two cell types, researchers from Pennsylvania State University, USA and Jinan University, China, have now developed a novel cell reprogramming strategy to transform proliferative glioma cells into non-proliferative neurons.
Giving further insights into their work published in Cancer Biology & Medicine, Prof. Gong Chen, lead scientist and corresponding author of the article, states, “Our reprogramming strategy principally differs from other anti-cancer therapies. We are not aiming to killing glioma cells, but rather change them into neurons. Therefore, potential side effects on other normal cells are low. Our study demonstrates successful reprogramming of glioma cells into neurons both in vitro and in vivo, using neural transcription factors.”
The team began by engineering GBM cells to express “transcription factors” that are known to trigger neuronal differentiation, like Neurogenic differentiation 1 (NeuroD1), Neurogenin-2 (Neurog2), and Achaete-scute homolog 1 (Ascl1). Interestingly, the reprogrammed glioma cells started looking more like neurons, and produced certain neuron-specific markers. This identity-change into neurons significantly decreased their division (or proliferation) rate.
The researchers found that each of the neural transcription factors induced cell conversion to a different neuronal type. Neurog2 expression yielded the fastest and most efficient cell conversion. Based on the neurotransmitter released, majority of the cells expressing Neurog2 and NeuroD1 became glutamatergic neurons, while those expressing Ascl1 became GABAergic neurons, underscoring specific signaling influences of each factor.
These differences were also accompanied by distinct gene expression patterns across the different neuronal subtypes, observed from RNA sequencing analysis, a technique used to study differentially expressed genes.
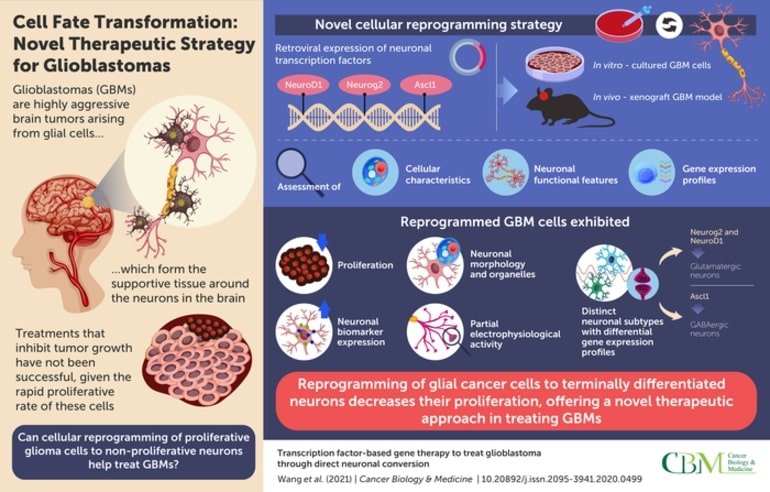
Next, they assessed the transformed glioma cells for their cellular and functional features. Notably, these converted cells exhibited an intra-cellular organelle arrangement similar to neurons and demonstrated neuronal signal transmission activity to a certain extent.
In order to validate their findings in vivo, the researchers injected retroviruses expressing the aforementioned factors into the brain of mice transplanted with GBM cells. Much to their delight, the GBM cells efficiently transformed into neuronal cells in the mice, evidenced by the expression of neuronal biomarkers. Moreover, cellular transformation also significantly inhibited the proliferation of these cells in the animals.
Together, these findings suggest that cellular reprogramming of glioma cells towards neuronal cell types, offers a promising therapeutic strategy that can slow down the growth of GBMs. Such a targeted approach can also help overcome the harmful side effects of conventional anti-cancer treatments on healthy brain cells.
Prof. Chen concludes by saying, “Our study opens a new direction for inhibiting the growth of gliomas. Future studies transitioning from rodents to non-human primates will help test whether we can use this reprogramming strategy to treat large gliomas in monkey brains. If successful, it will provide a promising treatment for millions of glioma patients worldwide.”
Cell fate transformation may indeed transform the fate of GBM patients as well!
About this brain cancer research news
Author: Indrani Das
Source: Cactus Communication
Contact: Indrani Das – Cactus Communications
Image: The image is credited to Cancer Biology and Medicine
Original Research: Closed access.
“Transcription factor-based gene therapy to treat glioblastoma through direct neuronal conversion” by Xin Wang et al. Cancer Biology and Medicine
Abstract
Transcription factor-based gene therapy to treat glioblastoma through direct neuronal conversion
Objective: Glioblastoma (GBM) is the most prevalent and aggressive adult primary cancer in the central nervous system. Therapeuticapproaches for GBM treatment are under intense investigation, including the use of emerging immunotherapies. Here, we proposean alternative approach to treat GBM through reprogramming proliferative GBM cells into non-proliferative neurons.
Methods: Retroviruses were used to target highly proliferative human GBM cells through overexpression of neural transcriptionfactors. Immunostaining, electrophysiological recording, and bulk RNA-seq were performed to investigate the mechanismsunderlying the neuronal conversion of human GBM cells. An in vivo intracranial xenograft mouse model was used to examine theneuronal conversion of human GBM cells.
Results: We report efficient neuronal conversion from human GBM cells by overexpressing single neural transcription factorNeurogenic differentiation 1 (NeuroD1), Neurogenin-2 (Neurog2), or Achaete-scute homolog 1 (Ascl1). Subtype characterizationshowed that the majority of Neurog2- and NeuroD1-converted neurons were glutamatergic, while Ascl1 favored GABAergicneuron generation. The GBM cell-converted neurons not only showed pan-neuronal markers but also exhibited neuron-specificelectrophysiological activities. Transcriptome analyses revealed that neuronal genes were activated in glioma cells after overexpressionof neural transcription factors, and different signaling pathways were activated by different neural transcription factors. Importantly,the neuronal conversion of GBM cells was accompanied by significant inhibition of GBM cell proliferation in both in vitro andin vivo models.
Conclusions: These results suggest that GBM cells can be reprogrammed into different subtypes of neurons, leading to a potentialalternative approach to treat brain tumors using in vivo cell conversion technology.