Summary: Disruptions in circulating white blood cells may be a key driver in ALSP, a rare form of early-onset dementia.
Source: TCD
Scientists at Trinity College Dublin today announced a significant advance in our understanding of an early-onset form of dementia that may also progress our understanding of conditions such as Alzheimer’s disease.
Adult onset Leukoencephalopathy with axonal Spheroids and Pigmented glia (ALSP) is an ultra-rare condition characterised by mutations in a gene called Colony stimulating factor-1 receptor (CSF1R). The condition manifests initially with psychiatric and behavioural changes in patients followed by a rapid progression of dementia in the third or fourth decade of life. While the condition is very rare, for affected families it can represent a devastating diagnosis.
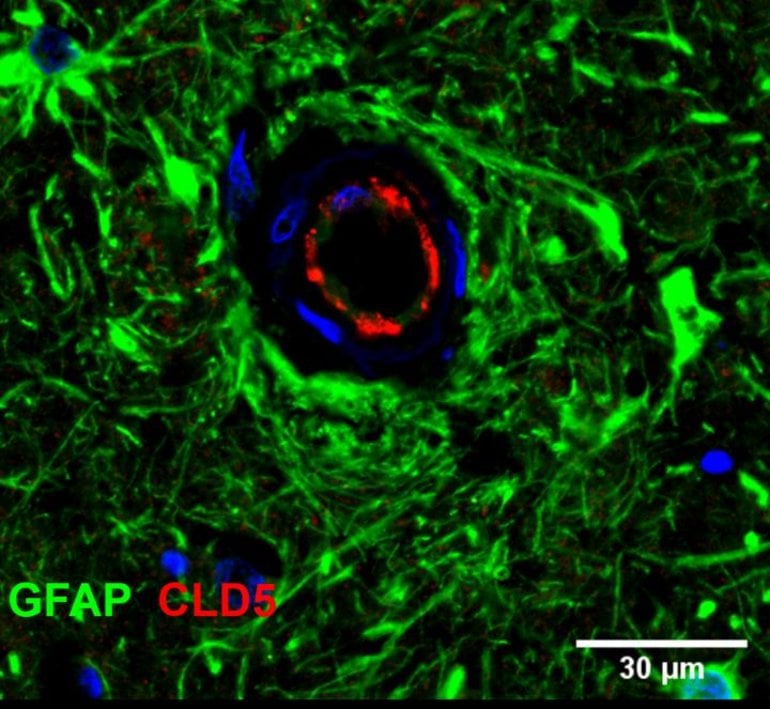
As the condition involves the degeneration of white matter in the brain, scientists previously thought that immune cells within the brain termed microglia were the primary culprits in driving pathology observed in this condition.
However, the Trinity team, working with patient samples as well as pre-clinical models, were able to show that dysfunctional circulating white blood cells were the key driver of neurodegeneration.
“This was fundamentally a translational research project, where data obtained from patient samples critically informed the direction of our pre-clinical studies. Our findings have shed light on a novel mechanism of neurodegeneration that may ultimately teach us more about common forms of dementia,” said Dr Matthew Campbell, Associate Professor at Trinity.
Importantly, the work has identified that a disruption in CSF1R function in patients, as well as in pre-clinical models, induces damage to the so-called blood-brain barrier (BBB). This damage can subsequently change the integrity of capillaries in the brain, causing them to leak and spark the deterioration of the brain. Intriguingly, dysfunctional white blood cells seem to be the main driver of this BBB breakdown.
Relevance to other dementias
Unfortunately, there are currently no approved therapies for conditions such as Alzheimer’s disease, which is in part due to the lack of a robust understanding of the early initiators of disease. If we can better understand what the early hallmarks of Alzheimer’s are, we may be able to develop novel forms of therapy that target these newly discovered mechanistic pathways.
Dr Conor Delaney, Irish Research Council scholar, Postdoctoral research fellow and first author of the study, added: “The most exciting aspect of our study is that we have now honed in on a novel pathway that to date has not been explored in great detail. Additionally, our data suggest that modifying white blood cell function may be therapeutically relevant for progressive neurodegenerative conditions.”
A multidisciplinary team of geneticists, immunologists, neurologists and neuropathologists from Trinity, the Royal College of Surgeons Ireland (RCSI), University College Cork (UCC), Sligo General Hospital and the University of Ghent undertook the study.
While shedding light on an often-neglected rare disease, the findings could pave the way for a targeted therapeutic approach for other forms of dementia.
Commenting on the clinical significance of the findings, Colin Doherty, Professor of Epilepsy in Trinity, said: “It is absolutely critical that we focus our research endeavours on identifying the underlying cause of neurodegenerative conditions. Studies like these will pave the way for better clinical management of our patients and hopefully new medicines to treat the condition.”
Funding: The research, published this week in the international journal, EMBO Molecular Medicine, was supported by the Irish Research Council (IRC), Science Foundation Ireland (SFI) – Future Neuro Centre, the St James’s Hospital foundation and the Ellen Mayston Bates bequest in the Trinity Foundation.
About this genetics and dementia research news
Source: TCD
Contact: Matthew Campbell – TCD
Image: The image is credited to TCD
Original Research: Open access.
“Attenuated CSF‐1R signalling drives cerebrovascular pathology” by Matthew Campbell et al. EMBO Molecular Medicine
Abstract
Attenuated CSF‐1R signalling drives cerebrovascular pathology
Cerebrovascular pathologies occur in up to 80% of cases of Alzheimer’s disease; however, the underlying mechanisms that lead to perivascular pathology and accompanying blood–brain barrier (BBB) disruption are still not fully understood. We have identified previously unreported mutations in colony stimulating factor‐1 receptor (CSF‐1R) in an ultra‐rare autosomal dominant condition termed adult‐onset leucoencephalopathy with axonal spheroids and pigmented glia (ALSP). Cerebrovascular pathologies such as cerebral amyloid angiopathy (CAA) and perivascular p‐Tau were some of the primary neuropathological features of this condition. We have identified two families with different dominant acting alleles with variants located in the kinase region of the CSF‐1R gene, which confer a lack of kinase activity and signalling. The protein product of this gene acts as the receptor for 2 cognate ligands, namely colony stimulating factor‐1 (CSF‐1) and interleukin‐34 (IL‐34). Here, we show that depletion in CSF‐1R signalling induces BBB disruption and decreases the phagocytic capacity of peripheral macrophages but not microglia. CSF‐1R signalling appears to be critical for macrophage and microglial activation, and macrophage localisation to amyloid appears reduced following the induction of Csf‐1rheterozygosity in macrophages. Finally, we show that endothelial/microglial crosstalk and concomitant attenuation of CSF‐1R signalling causes re‐modelling of BBB‐associated tight junctions and suggest that regulating BBB integrity and systemic macrophage recruitment to the brain may be therapeutically relevant in ALSP and other Alzheimer’s‐like dementias.






