Summary: Researchers have discovered a way yo simultaneously monitor the switching on and off of clock genes in mice and the effects on their behavior in real time.
Source: Hokkaido Univeristy.
Scientists have found a way to simultaneously monitor the switching on and off of circadian “clock” genes and their effects on mouse behaviour in real-time.
“Clock genes” turn on and off, or “express”, in rhythmic patterns throughout the body to regulate physiological conditions and behaviour. When and how these genes express, especially in tissues outside the brain, is still poorly understood. Until now, scientists have lacked sufficient means to simultaneously monitor gene rhythms in specific tissues in freely moving subjects.
Bioluminescence is a technique that often involves modifying certain genes. When the target gene is switched on, it also expresses an inserted gene, leading to the emission of a light signal. Every time the target gene turns on, light is emitted. This technique has been successfully used to monitor gene expression in fully anaesthetized mice. However, anaesthesia is believed to affect the expression of clock genes. Other techniques not requiring anaesthetization have their own limitations.
A team of scientists from Hokkaido University in Japan developed a new imaging technique that allowed them to monitor the expression of clock genes, including the per1 gene, in multiple tissues in moving fully conscious mice. When per1, which is known to be important for maintaining circadian rhythms, turns on in the transgenic mice, a light signal is emitted.
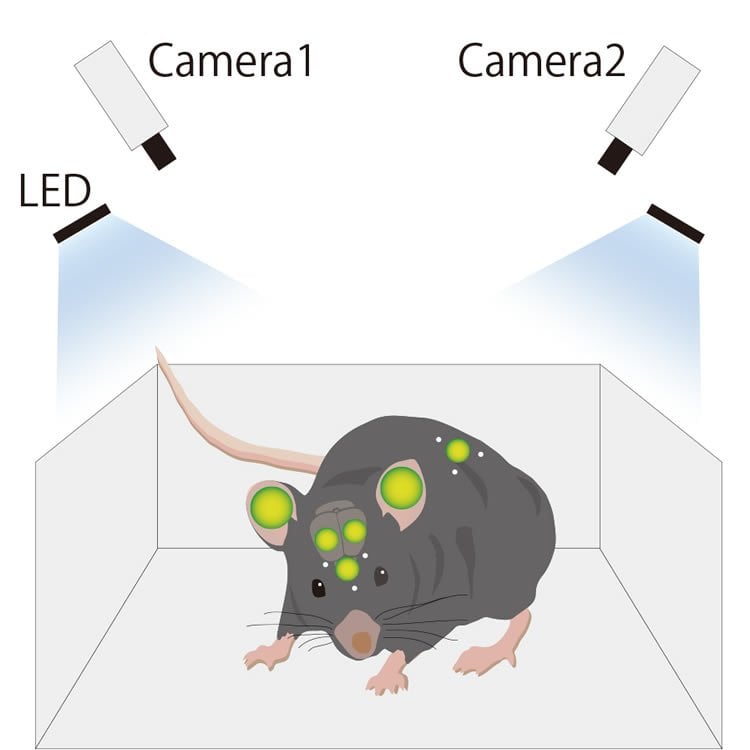
To track the movement of the mouse, scintillators that fluoresce were attached to the back and head of the mice. The team developed a software program that was able to detect the three-dimensional position of the scintillators in the freely moving mice based on images received from two separate cameras placed in the mouse cage. The team also developed a set of algorithms that allowed them to identify the intensity of the bioluminescent signals from target tissues (olfactory bulb, right and left ears and cortex, skin) despite the movement of the mice.
“Using the present system, we observed robust circadian rhythmicity in per1 expression at six different areas in the bodies of freely moving mice,” the researchers report in their study published in the journal Nature Communications. Per1 expression was at its peak in all six areas at the onset of mouse daily activity. When the Hokkaido team artificially shifted the hours of night and day for the mice, such as might happen when shifting time zones, per1’s rhythmic expression became desynchronized for one day in the different areas and then synchronized again.
While further improvements are still required, the new monitoring technique could be widely applied to many areas of biomedical research, as well as in applications beyond medicine, the researchers say.
Funding: This work was supported by Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Source: Hokkaido Univeristy
Image Source: This NeuroscienceNews.com image is adapted from the Hokkaido Univeristy press release.
Original Research: Full open access research for “In vivo imaging of clock gene expression in multiple tissues of freely moving mice” by Toshiyuki Hamada, Kenneth Sutherland, Masayori Ishikawa, Naoki Miyamoto, Sato Honma, Hiroki Shirato and Ken-ichi Honma in Nature Communications. Published online June 10 2016 doi:10.1038/ncomms11705
[cbtabs][cbtab title=”MLA”]Hokkaido Univeristy. “Watching The Luminescent Gene Switch.” NeuroscienceNews. NeuroscienceNews, 20 June 2016.
<https://neurosciencenews.com/clock-gene-optogenetics-4528/>.[/cbtab][cbtab title=”APA”]Hokkaido Univeristy. (2016, June 20). Watching The Luminescent Gene Switch. NeuroscienceNews. Retrieved June 20, 2016 from https://neurosciencenews.com/clock-gene-optogenetics-4528/[/cbtab][cbtab title=”Chicago”]Hokkaido Univeristy. “Watching The Luminescent Gene Switch.” https://neurosciencenews.com/clock-gene-optogenetics-4528/ (accessed June 20, 2016).[/cbtab][/cbtabs]
Abstract
In vivo imaging of clock gene expression in multiple tissues of freely moving mice
Clock genes are expressed throughout the body, although how they oscillate in unrestrained animals is not known. Here, we show an in vivo imaging technique that enables long-term simultaneous imaging of multiple tissues. We use dual-focal 3D tracking and signal-intensity calibration to follow gene expression in a target area. We measure circadian rhythms of clock genes in the olfactory bulb, right and left ears and cortices, and the skin. In addition, the kinetic relationship between gene expression and physiological responses to experimental cues is monitored. Under stable conditions gene expression is in phase in all tissues. In response to a long-duration light pulse, the olfactory bulb shifts faster than other tissues. In Cry1−/− Cry2−/− arrhythmic mice circadian oscillation is absent in all tissues. Thus, our system successfully tracks circadian rhythms in clock genes in multiple tissues in unrestrained mice.
“In vivo imaging of clock gene expression in multiple tissues of freely moving mice” by Toshiyuki Hamada, Kenneth Sutherland, Masayori Ishikawa, Naoki Miyamoto, Sato Honma, Hiroki Shirato and Ken-ichi Honma in Nature Communications. Published online June 10 2016 doi:10.1038/ncomms11705






