Summary: Researchers uncovered a crucial link between the gene Angiogenin (ANG) and age-related neurodegenerative diseases such as frontotemporal dementia (FTD), motor neuron disease (MND), and Parkinson’s disease. In its mutated form, ANG causes stem cells to delay their differentiation into specialized nerve cells, leading to neurodevelopmental defects.
The research also revealed that the healthy form of ANG protects nerve cells, while mutations make them more vulnerable to stress and premature death. This discovery offers new insights into early intervention possibilities for these debilitating diseases.
Key Facts:
- The ANG gene, associated with neurodegenerative diseases, affects the development of nerve cells.
- Mutated ANG delays differentiation of stem cells, leading to neurodevelopmental defects.
- Understanding ANG’s role could lead to early intervention and gene therapy for disease prevention.
Source: University of Bath
New understanding of a gene that is linked to some forms of dementia and other age-related diseases gives scientists fresh hope that action can be taken against these diseases long before the onset of symptoms.
The gene – called Angiogenin or ANG – is associated with a number of neurodegenerative diseases commonly associated with old age, including frontotemporal dementia (FTD), motor neuron disease (MND) and Parkinson’s disease.
In their latest research, scientists at the University of Bath have found this gene – in its healthy state – plays an important role in the pace at which undifferentiated stem cells develop into specialised nerve cells.
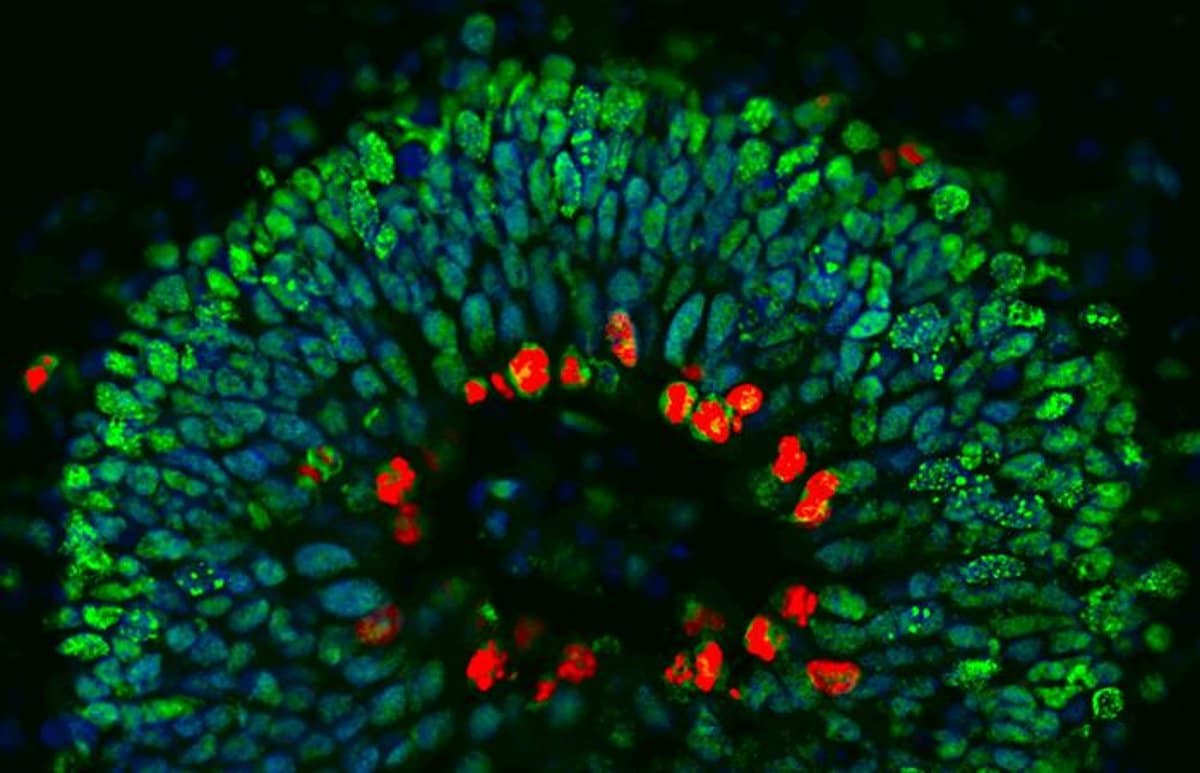
In its mutated form, ANG causes stem cells to persist in their original state longer than they should. In lab experiments, this slowing down of the differentiation process was seen to result in striking neurodevelopmental defects in nerve cells once they had reached their adult form.
“This suggests nerve-cell degeneration may be primed by defects occurring during early development,” said Dr Vasanta Subramanian, who led the research from the Department of Life Sciences.
The study is published this month in the Journal of Pathology.
In earlier work, the same Bath research group found that ANG, in its healthy form, protects nerve cells against damage, degeneration and impairment of function. By contrast, the mutated form of the gene causes nerve cells to be more susceptible to stress (a natural occurrence as cells age and experience wear and tear), leading to premature cell death.
“This new discovery adds to our understanding of Angiogenin and its importance in protecting us from diseases associated with aging,” said Dr Subramanian.
Mini-brains grown in the lab
For their latest study, the researchers studied a family affected by both frontotemporal dementia and motor neuron disease. Genetic tests showed that some family members had mutations in Angiogenin while others did not.
For all family members, ‘mini-brains’ were grown in the lab. A mini-brain is a tiny 3D structure grown from clusters of human stem cells. It provides scientists with a realistic model to study the step-by-step development of disease. It also provides an ideal structure on which to screen drugs.
The researchers observed striking neurodevelopmental defects in the mini-brains of family members carrying the ANG mutation.
“This seems to indicate that subtle development defects play a role in disease susceptibility or onset,” said Dr Subramanian.
She added: “I envisage a time when we will be identifying people who are susceptible to these diseases, screening them for genetic mutations and offering early-intervention gene therapy to fix the defects.”
Dr Subramanian said more research was needed to elucidate the mechanisms by which ANG acts to protect cells and to better understand its function in stem cells.
This work was funded by grants from the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs), BRACE and the Wellcome Trust VIP award.
BRACE CEO, Chris Williams, said: “We applaud Dr Subramanian’s innovative research, which could make a big difference in tackling frontotemporal dementia. Better understanding of the Angiogenin gene and its link to FTD could support treatment to slow down or stop the disease in the future.
“This type of dementia tends to have an early onset between the ages of 45-65 years, and often has a devastating impact during middle age. We are hopeful that this BRACE funded research may play a key role in one day reducing the impact of the condition.”
Dr Jessica Eddy, NC3Rs Regional Programme Manager, said: “Research into the brain and neurological disorders relies in large part on animal models and it is fantastic to see Vasanta’s mini-brain ‘organoids’ delivering new insights into neurodegenerative diseases.
“It is testament to the utility of these models that they are still being applied to new research questions, almost 15 years after we awarded Vasanta the initial funding to develop human cell-based alternatives to the use of animals in ALS (the most common form of motor neuron disease) research.”
About this neurodegeneration and genetics research news
Author: Chris Melvin
Source: University of Bath
Contact: Chris Melvin – University of Bath
Image: The image is credited to Ross Ferguson and Vasanta Subramanian
Original Research: Open access.
“Neural stem cell homeostasis is affected in cortical organoids carrying a mutation in Angiogenin” by Vasanta Subramanian et al. Journal of Pathology
Abstract
Neural stem cell homeostasis is affected in cortical organoids carrying a mutation in Angiogenin
Mutations in Angiogenin (ANG) and TARDBP encoding the 43 kDa transactive response DNA binding protein (TDP-43) are associated with amyotrophic lateral sclerosis and frontotemporal dementia (ALS-FTD).
ANG is neuroprotective and plays a role in stem cell dynamics in the haematopoietic system. We obtained skin fibroblasts from members of an ALS-FTD family, one with mutation in ANG, one with mutation in both TARDBP and ANG, and one with neither mutation.
We reprogrammed these fibroblasts to induced pluripotent stem cells (iPSCs) and generated cortical organoids as well as induced stage-wise differentiation of the iPSCs to neurons.
Using these two approaches we investigated the effects of FTD-associated mutations in ANG and TARDBP on neural precursor cells, neural differentiation, and response to stress.
We observed striking neurodevelopmental defects such as abnormal and persistent rosettes in the organoids accompanied by increased self-renewal of neural precursor cells.
There was also a propensity for differentiation to later-born neurons. In addition, cortical neurons showed increased susceptibility to stress, which is exacerbated in neurons carrying mutations in both ANG and TARDBP. The cortical organoids and neurons generated from patient-derived iPSCs carrying ANG and TARDBP gene variants recapitulate dysfunctions characteristic of frontotemporal lobar degeneration observed in FTD patients.
These dysfunctions were ameliorated upon treatment with wild type ANG. In addition to its well-established role during the stress response of mature neurons, ANG also appears to play a role in neural progenitor dynamics.
This has implications for neurogenesis and may indicate that subtle developmental defects play a role in disease susceptibility or onset.






