Summary: A new study reveals how AI-driven deep learning models can decode the genetic regulatory switches that define brain cell types across species. By analyzing human, mouse, and chicken brains, researchers found that some brain cell types remain highly conserved over 320 million years, while others have evolved uniquely.
This regulatory code not only sheds light on brain evolution but also provides new tools for studying gene regulation in health and disease. The findings highlight how AI can identify preserved and divergent genetic instructions controlling brain function across species.
The study also has implications for understanding neurological disorders by linking genetic variants to cognitive traits. Researchers are now expanding their models to study the brains of various animals and human disease states like Parkinson’s.
Key Facts
- Evolutionary Conservation: Some brain cell regulatory codes have remained unchanged for over 320 million years.
- AI-Powered Discovery: Deep learning models help decode genetic regulation across species.
- Disease Applications: These models could be used to study neurological disorders like Parkinson’s.
Source: VIB
In a new study published in Science, a Belgian research team explores how genetic switches controlling gene activity define brain cell types across species.
They trained deep learning models on human, mouse, and chicken brain data and found that while some cell types are highly conserved between birds and mammals after millions of years of evolution, others have evolved differently.

The findings not only shed new light on brain evolution; they also provide powerful tools for studying how gene regulation shapes different cell types, across species or different disease states.
Our brain, and by extension our entire body, is made up of many different types of cells. While they share the same DNA, all these cell types have their own shape and function.
What makes each cell type different is a complex puzzle that researchers have been trying to put together for decades from short DNA sequences that act like switches, controlling which genes are turned on or off.
The fine-tuned regulation of these switches ensures that each type of brain cell uses just the right genetic instructions from the genome to perform its unique role. Scientists refer to the unique patterns of these genetic switches as a regulatory code.
AI to crack the code
Prof. Stein Aerts and his team at VIB.AI and the VIB-KU Leuven Center for Brain & Disease Research study the fundamental principles of this regulatory code, and how it may impact diseases such as cancer or brain disorders.
They develop deep learning methods to help make sense of the huge amount of information on gene regulation they gather from thousands and thousands of individual cells.
“Deep-learning models working with the DNA sequence code have helped us enormously to identify regulatory mechanisms across different cell types,” explains Aerts.
“Now, we wanted to explore whether this regulatory code could also inform us on how these cell types are conserved across species.”
One example of where such a question is highly relevant is in the brain. Despite shared developmental trajectories, the brains of mammals and birds display a strikingly different neuroanatomy.
Aerts and his team have now applied deep learning models to assess whether the existing differences and similarities are reflected in shared or divergent regulatory codes.
Tool to study evolution
Nikolai Hecker and Niklas Kempynck, respectively postdoc and PhD student in the Aerts lab, developed and implemented machine learning models to characterize and compare different types of cells across human, mouse, and chicken brains, covering approximately 320 million years of evolution.
But before they could truly compare, they first had to better understand the cell type composition of the chicken brain, so they created a comprehensive transcriptomic atlas.
“Our study demonstrates how we can use deep learning to characterize and compare different cell types based on their regulatory codes,” explains Hecker. “We can use these codes to compare genomes of different species, identify which regulatory codes have been evolutionarily preserved, and gain insights into how cell types have evolved.”
The team found that while some regulatory cell type codes are highly conserved between birds and mammals, others have evolved differently. Notably, the regulatory codes for certain bird neurons resemble those of deep-layer neurons in the mammalian neocortex.
“Looking directly at the regulatory code presents a significant advantage,” adds Kempynck, “It can tell us which regulatory principles are shared across species, even if the DNA sequence itself has changed.”
Tool to study disease
This regulatory information is useful beyond understanding evolution. In previous work, Aerts and his team already verified that regulatory codes for melanoma (skin cancer) cell states are conserved between mammals and zebrafish. They also identified variants in the genomes of melanoma patients.
The models presented in the current study on brain cell types provide useful tools to study the impact of genomic variants and their association with mental or cognitive traits and disorders.
Aerts: “Ultimately, models that learn the genomic regulatory code hold the potential to screen genomes and investigate the presence or absence of specific cell types or cell states in any species. This would be a powerful tool to study and better understand disease.”
To the zoo
Aerts and his team are already applying their models on both fronts, he says:
“In collaboration with Zoo Science and Wildlife Rescue Center, we are now expanding our evolutionary modeling to many more animal brains: different types of fish to dear, hedgehogs and capibaras. At the same time, we’re also exploring how these AI models can help to unravel genetic variation linked to Parkinson’s disease.”
About this genetics, AI, and evolutionary neuroscience research news
Author: Gunnar De Winter
Source: VIB
Contact: Gunnar De Winter – VIB
Image: The image is credited to Neuroscience News
Original Research: Closed access.
“Enhancer-driven cell type comparison reveals similarities between the mammalian and bird pallium” by Stein Aerts et al. Science
Abstract
Enhancer-driven cell type comparison reveals similarities between the mammalian and bird pallium
INTRODUCTION
The identity of cell types is governed by gene regulatory networks, which comprise cell type–specific combinations of transcription factors (TFs) that bind to genomic enhancer regions.
The arrangements of TF binding sites form cell type–specific enhancer codes. Deep learning models trained on single-cell data provide the means to model and characterize enhancer codes at nucleotide resolution. Enhancer codes at such resolution have not yet been characterized for the mammalian telencephalon, which constitutes a major part of the forebrain, including the pallium.
The pallium displays notable neuroanatomical differences between mammals and nonmammalian vertebrates. Most noticeable, the mammalian pallium contains a six-layered neocortex that is absent in all nonmammalian vertebrates, such as birds.
Homologies between the mammalian and bird pallium are subject to a decades-long debate. It is currently unknown whether enhancer codes are conserved across vertebrate brains and whether they are informative to resolve homology relationships between species at the cell type level.
RATIONALE
To characterize and compare the enhancer codes of brain cell types between the mammalian and bird pallium, we generated single-cell multiome (scMultiome) and spatially resolved transcriptomics data of the chicken telencephalon.
As a baseline to map cell type similarities between species, we compared the transcriptomes of telencephalon cell types between human, mouse, and chicken. We then used variable chromatin accessibility as a proxy to identify potential genomic enhancer regions and to assess their cell type specificity.
Next, we trained sequence-based deep learning models on these regions to infer cell type–specific enhancer codes for the human, mouse, and chicken telencephalon. We implemented three metrics that exploit enhancer codes to compare cell types between species.
RESULTS
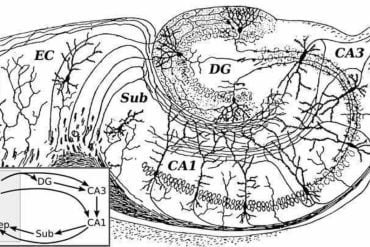
Excitatory neurons of the chicken telencephalon distinctly localize to pallial neuroanatomical regions, including the mesopallium, entopallium, hyperpallium, and nidopallium.
Based on the transcriptomic and enhancer code comparisons, nonneuronal and γ-aminobutyric acid–mediated (GABAergic) cell types show a high degree of similarity across birds and mammals, which is reflected by conserved TF combinations for these cell types.
On the other hand, the enhancer codes of excitatory neurons in the mammalian and avian pallium exhibit a higher degree of divergence. These matches only partially agree with existing evolutionary models for homologies between vertebrate pallial cell types based on developmental trajectories and brain circuitry.
We found that the mammalian deep-layer excitatory neurons are most similar to mesopallial neurons, and mammalian neocortical upper-layer, piriform cortex, and amygdalar neurons are most similar to hyper- and nidopallial neurons.
As a validation for the predicted correspondences between mammalian and bird cell types, we performed in vivo enhancer reporter assays. We show that chicken enhancer sequences exhibit activity in the corresponding mammalian telencephalic cell types when assayed in mouse brains.
CONCLUSION
Our study shows that enhancer codes can be exploited to infer cell type correspondences between species that are in line with transcriptomic comparisons.
Joint comparisons of transcriptomes and deep learning–based enhancer codes reveal both expected and unexpected correspondences between cell types in the mammalian and avian telencephalon, indicating conserved regulatory programs that likely originated in the common amniote ancestor and have been co-opted or diversified.
The proposed enhancer code–based approaches are generally applicable and can be used to characterize and compare cell types across species using the genomic regulatory code.