Summary: A new study compares genetic influences in families of children diagnosed with autism.
Source: Broad Institute.
A new study of inherited genetic risk indicates that common genetic variations throughout the genome act in addition to rare, deleterious mutations in autism-associated genes to create risk for autism.
Studies of genetic risk for developing autism spectrum disorder (ASD) often compare DNA from those diagnosed with autism to that of neurotypical controls, but these approaches can be confounded by external factors. To get a clearer look at the genetic underpinnings of autism risk, a team led by researchers from the Broad Institute’s Stanley Center for Psychiatric Research and the Analytic and Translational Genetics Unit at Massachusetts General Hospital and Harvard Medical School took a new approach, published in Nature Genetics, comparing genetic influences within families in which a child has been diagnosed with autism.
In the human genome, common genetic variations can each contribute a small level of risk for developing a given disorder. These variants can be aggregated to create a “polygenic risk score,” which represents part of an individual’s overall disorder risk.
In this study, Elise Robinson, an associated scientist at Broad and assistant professor of epidemiology at the Harvard T.H. Chan School of Public Health, first author Daniel Weiner, a scientist in Robinson’s lab, and colleagues calculated polygenic risk scores for developing autism in members of 6,454 families with one or more children diagnosed with ASD. The risk scores were based on the participants’ individual genotypes compared against data from genome-wide association studies.
On average, children’s genetic risk scores for any phenotype equal an average of their parents’ scores. However, the team discovered that children with ASD have a higher risk score on average for developing ASD — meaning that more of the contributing common variants from the parents have been inherited together, versus what would be expected if the risk score was an average of the parents’. Children with ASD in the study were also likely to have independently over-inherited their parents’ polygenic risk for developing schizophrenia, as well as polygenic influences associated with more years of education (which are strongly correlated with the polygenic influences on IQ score).
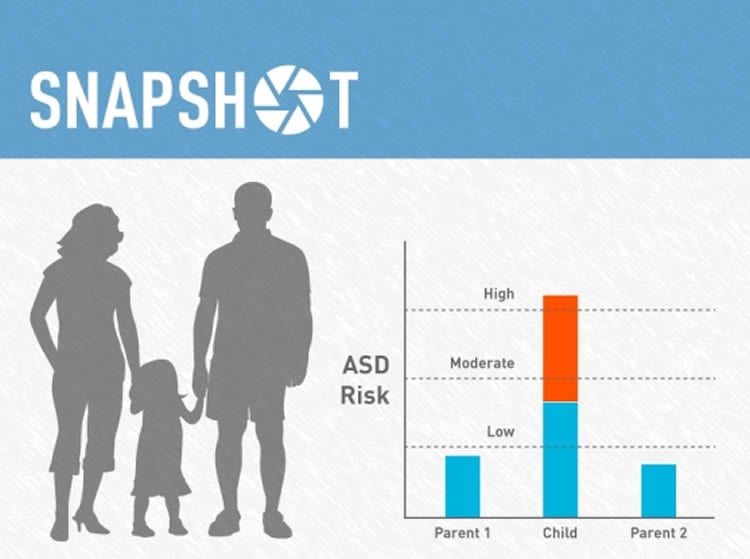
The independent inheritance of these influences may help to explain the different ways that autism can manifest. For example, higher polygenic risk for schizophrenia was associated with lower IQ , whereas higher polygenic scores for years of education were associated with higher IQ, in children with ASD.
The team also found that risk for developing ASD is increased beyond the common polygenic risk score if a rare, harmful mutation, newly arising in the child, occurs in an autism-associated gene. In children with ASD, these rare variants are associated with more severe neurodevelopmental impacts, such as intellectual disability, seizures, and motor function delay.
The study results point to multiple types of genetic risk for ASD, highlighting the need for a better understanding of the behavioral and cognitive traits associated with autism in order to eventually develop models or therapies. Using genetic data from parents and their children to dissect the roots of a disorder also eliminates many potential confounding factors, and the research team noted the utility of this analytic method for studying other types of polygenic risk.
Source: Karen Zusi – Broad Institute
Image Source: NeuroscienceNews.com image is credited to Susanna M. Hamilton, Broad Communications.
Original Research: Abstract for “Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders” by Daniel J Weiner, Emilie M Wigdor, Stephan Ripke, Raymond K Walters, Jack A Kosmicki, Jakob Grove, Kaitlin E Samocha, Jacqueline I Goldstein, Aysu Okbay, Jonas Bybjerg-Grauholm, Thomas Werge, David M Hougaard, Jacob Taylor, iPSYCH-Broad Autism Group, Marie Bækvad-Hansen, Ashley Dumont, Christine Hansen, Thomas F Hansen, Daniel Howrigan, Manuel Mattheisen, Jennifer Moran, Ole Mors, Merete Nordentoft, Bent Nørgaard-Pedersen, Timothy Poterba, Jesper Poulsen, Christine Stevens, Psychiatric Genomics Consortium Autism Group, Verneri Anttila, Peter Holmans, Hailiang Huang, Lambertus Klei, Phil H Lee, Sarah E Medland, Benjamin Neale, Lauren A Weiss, Lonnie Zwaigenbaum, Timothy W Yu, Kerstin Wittemeyer, A Jeremy Willsey, Ellen M Wijsman, Thomas H Wassink, Regina Waltes, Christopher A Walsh, Simon Wallace, Jacob A S Vorstman, Veronica J Vieland, Astrid M Vicente, Herman van Engeland, Kathryn Tsang, Ann P Thompson, Peter Szatmari, Oscar Svantesson, Stacy Steinberg, Kari Stefansson, Hreinn Stefansson, Matthew W State, Latha Soorya, Teimuraz Silagadze, Stephen W Scherer, Gerard D Schellenberg, Sven Sandin, Evald Saemundsen, Guy A Rouleau, Bernadette Rogé, Kathryn Roeder, Wendy Roberts, Jennifer Reichert, Abraham Reichenberg, Karola Rehnström, Regina Regan, Fritz Poustka, Christopher S Poultney, Joseph Piven, Dalila Pinto, Margaret A Pericak-Vance, Milica Pejovic-Milovancevic, Marianne G Pedersen, Carsten B Pedersen, Andrew D Paterson, Jeremy R Parr, Alistair T Pagnamenta, Guiomar Oliveira, John I Nurnberger, Merete Nordentoft, Michael T Murtha, Susana Mouga, Ole Mors, Eric M Morrow, Daniel Moreno De Luca, Anthony P Monaco, Nancy Minshew, Alison Merikangas, William M McMahon, Susan G McGrew, Manuel Mattheisen, Igor Martsenkovsky, Donna M Martin, Shrikant M Mane, Pall Magnusson, Catherine Lord, Jonathan M Green, Bridget Fernandez, Bernie Devlin, Patrícia B S Celestino-Soper, Nadia Bolshakova, Cátia Café, Hakon Hakonarson, David Amaral, Elena Bacchelli, Sabine M Klauck, Marion Leboyer, Hilary Coon, Patrick F Bolton, Arthur L Beaudet, Richard Anney, Joachim Hallmayer, Sean Ennis, David H Ledbetter, Pat Levitt, M Daniele Fallin, Agatino Battaglia, Michael L Cuccaro, Anders D Børglum, Maretha V De Jonge, Aarno Palotie, Raphael Bernier, Silvia De Rubeis, Frederico Duque, Preben Bo Mortensen, Daniel H Geschwind, Dan E Arking, Irva Hertz-Picciotto, Jennifer K Lowe, Stephan J Sanders, Judith Conroy, Alexander Kolevzon, John Gilbert, Anthony J Bailey, Christopher Gillberg, Vanessa H Bal, Ann S Le Couteur, Marie Bækvad-Hansen, Christina M Hultman, Jillian Casey, Robert Hendren, Christa Lese Martin, Stephen J Guter, Aravinda Chakravarti, David Skuse, Gillian Baird, Sean Brennan, George Davey Smith, Tiago Magalhaes, Rita M Cantor, Somer Bishop, Joel S Bader, Edwin H Cook, Joseph D Buxbaum, Catalina Betancur, Inês C Conceição, Suma Jacob, A Gulhan Ercan-Sencicek, Catarina T Correia, Susan E Folstein, Jonathan L Haines, Christine M Freitag, Thomas Bourgeron, Susan Santangelo, Bozenna Iliadou, Evdokia Anagnostou, Mark J Daly, Michael Gill, Sven Bölte, Elena Maestrini, Andreas G Chiocchetti, Joana Almeida, Christine Ladd-Acosta, Richard Delorme, Eric Fombonne, Louise Gallagher, Dorothy E Grice, Geraldine Dawson, Christine S Hansen, Elise B Robinson, Arthur P Goldberg, James S Sutcliffe, Andrew Green & Eftichia Duketis in Nature Genetics. Published online May 15 2017 doi:10.1038/ng.3863
[cbtabs][cbtab title=”MLA”]Broad Institute “The Genetic Architecture of Risk For Autism Spectrum Disorder.” NeuroscienceNews. NeuroscienceNews, 16 May 2017.
<https://neurosciencenews.com/asd-genetic-risk-6689/>.[/cbtab][cbtab title=”APA”]Broad Institute (2017, May 16). The Genetic Architecture of Risk For Autism Spectrum Disorder. NeuroscienceNew. Retrieved May 16, 2017 from https://neurosciencenews.com/asd-genetic-risk-6689/[/cbtab][cbtab title=”Chicago”]Broad Institute “The Genetic Architecture of Risk For Autism Spectrum Disorder.” https://neurosciencenews.com/asd-genetic-risk-6689/ (accessed May 16, 2017).[/cbtab][/cbtabs]
Abstract
Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders
Autism spectrum disorder (ASD) risk is influenced by common polygenic and de novo variation. We aimed to clarify the influence of polygenic risk for ASD and to identify subgroups of ASD cases, including those with strongly acting de novo variants, in which polygenic risk is relevant. Using a novel approach called the polygenic transmission disequilibrium test and data from 6,454 families with a child with ASD, we show that polygenic risk for ASD, schizophrenia, and greater educational attainment is over-transmitted to children with ASD. These findings hold independent of proband IQ. We find that polygenic variation contributes additively to risk in ASD cases who carry a strongly acting de novo variant. Lastly, we show that elements of polygenic risk are independent and differ in their relationship with phenotype. These results confirm that the genetic influences on ASD are additive and suggest that they create risk through at least partially distinct etiologic pathways.
“Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders” by Daniel J Weiner, Emilie M Wigdor, Stephan Ripke, Raymond K Walters, Jack A Kosmicki, Jakob Grove, Kaitlin E Samocha, Jacqueline I Goldstein, Aysu Okbay, Jonas Bybjerg-Grauholm, Thomas Werge, David M Hougaard, Jacob Taylor, iPSYCH-Broad Autism Group, Marie Bækvad-Hansen, Ashley Dumont, Christine Hansen, Thomas F Hansen, Daniel Howrigan, Manuel Mattheisen, Jennifer Moran, Ole Mors, Merete Nordentoft, Bent Nørgaard-Pedersen, Timothy Poterba, Jesper Poulsen, Christine Stevens, Psychiatric Genomics Consortium Autism Group, Verneri Anttila, Peter Holmans, Hailiang Huang, Lambertus Klei, Phil H Lee, Sarah E Medland, Benjamin Neale, Lauren A Weiss, Lonnie Zwaigenbaum, Timothy W Yu, Kerstin Wittemeyer, A Jeremy Willsey, Ellen M Wijsman, Thomas H Wassink, Regina Waltes, Christopher A Walsh, Simon Wallace, Jacob A S Vorstman, Veronica J Vieland, Astrid M Vicente, Herman van Engeland, Kathryn Tsang, Ann P Thompson, Peter Szatmari, Oscar Svantesson, Stacy Steinberg, Kari Stefansson, Hreinn Stefansson, Matthew W State, Latha Soorya, Teimuraz Silagadze, Stephen W Scherer, Gerard D Schellenberg, Sven Sandin, Evald Saemundsen, Guy A Rouleau, Bernadette Rogé, Kathryn Roeder, Wendy Roberts, Jennifer Reichert, Abraham Reichenberg, Karola Rehnström, Regina Regan, Fritz Poustka, Christopher S Poultney, Joseph Piven, Dalila Pinto, Margaret A Pericak-Vance, Milica Pejovic-Milovancevic, Marianne G Pedersen, Carsten B Pedersen, Andrew D Paterson, Jeremy R Parr, Alistair T Pagnamenta, Guiomar Oliveira, John I Nurnberger, Merete Nordentoft, Michael T Murtha, Susana Mouga, Ole Mors, Eric M Morrow, Daniel Moreno De Luca, Anthony P Monaco, Nancy Minshew, Alison Merikangas, William M McMahon, Susan G McGrew, Manuel Mattheisen, Igor Martsenkovsky, Donna M Martin, Shrikant M Mane, Pall Magnusson, Catherine Lord, Jonathan M Green, Bridget Fernandez, Bernie Devlin, Patrícia B S Celestino-Soper, Nadia Bolshakova, Cátia Café, Hakon Hakonarson, David Amaral, Elena Bacchelli, Sabine M Klauck, Marion Leboyer, Hilary Coon, Patrick F Bolton, Arthur L Beaudet, Richard Anney, Joachim Hallmayer, Sean Ennis, David H Ledbetter, Pat Levitt, M Daniele Fallin, Agatino Battaglia, Michael L Cuccaro, Anders D Børglum, Maretha V De Jonge, Aarno Palotie, Raphael Bernier, Silvia De Rubeis, Frederico Duque, Preben Bo Mortensen, Daniel H Geschwind, Dan E Arking, Irva Hertz-Picciotto, Jennifer K Lowe, Stephan J Sanders, Judith Conroy, Alexander Kolevzon, John Gilbert, Anthony J Bailey, Christopher Gillberg, Vanessa H Bal, Ann S Le Couteur, Marie Bækvad-Hansen, Christina M Hultman, Jillian Casey, Robert Hendren, Christa Lese Martin, Stephen J Guter, Aravinda Chakravarti, David Skuse, Gillian Baird, Sean Brennan, George Davey Smith, Tiago Magalhaes, Rita M Cantor, Somer Bishop, Joel S Bader, Edwin H Cook, Joseph D Buxbaum, Catalina Betancur, Inês C Conceição, Suma Jacob, A Gulhan Ercan-Sencicek, Catarina T Correia, Susan E Folstein, Jonathan L Haines, Christine M Freitag, Thomas Bourgeron, Susan Santangelo, Bozenna Iliadou, Evdokia Anagnostou, Mark J Daly, Michael Gill, Sven Bölte, Elena Maestrini, Andreas G Chiocchetti, Joana Almeida, Christine Ladd-Acosta, Richard Delorme, Eric Fombonne, Louise Gallagher, Dorothy E Grice, Geraldine Dawson, Christine S Hansen, Elise B Robinson, Arthur P Goldberg, James S Sutcliffe, Andrew Green & Eftichia Duketis in Nature Genetics. Published online May 15 2017 doi:10.1038/ng.3863






