Summary: Brain stimulation resulted in progressive alterations to brain plasticity.
Source: SfN
Recordings of neural activity during therapeutic stimulation can be used to predict subsequent changes in brain connectivity, according to a study of epilepsy patients published in Journal of Neuroscience. This approach could inform efforts to improve brain stimulation treatments for depression and other psychiatric disorders.
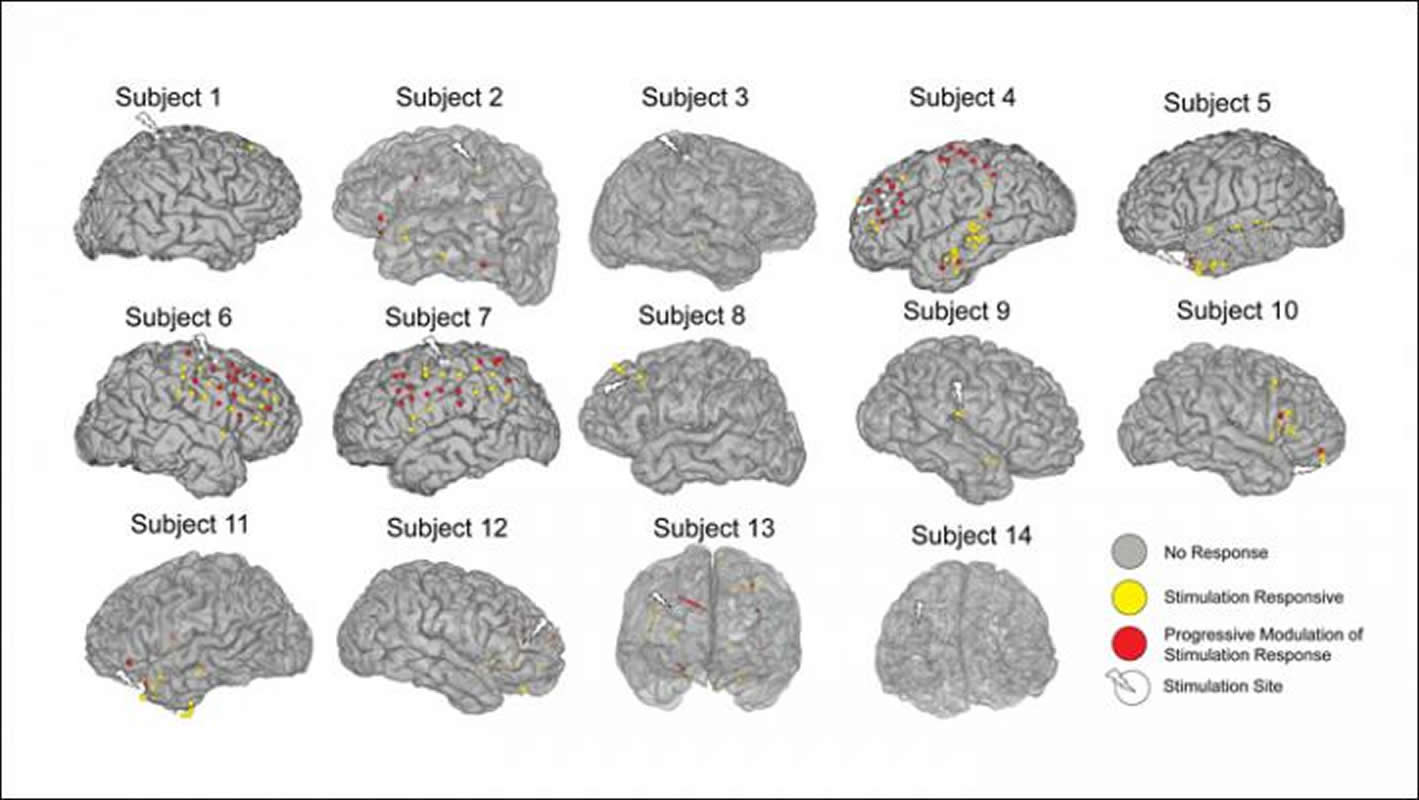
Corey Keller and colleagues delivered electrical stimulation from implanted electrodes in 14 patients while recording participants’ brain activity. Repeated sets of stimulation resulted in progressive changes to the brain’s response to simulation, with stronger responses in brain regions connected to the stimulation site. The researchers observed these changes in a matter of minutes, suggesting that electrical stimulation induces the brain to rapidly reorganize itself.
Assessing brain activity before, during, and after stimulation has the potential to personalize neuromodulation therapies. Whether these results will translate to non-invasive techniques, such as transcranial magnetic stimulation, and to other patient populations remains to be determined.
Source:
SfN
Media Contacts:
David Barnstone – SfN
Image Source:
The image is credited to Huang et al., JNeurosci (2019).
Original Research: Closed access
“Intracortical dynamics underlying repetitive stimulation predicts changes in network connectivity”. Yuhao Huang, Boglárka Hajnal, László Entz, Dániel Fabó, Jose L. Herrero, Ashesh D. Mehta and Corey J. Keller.
Journal of Neuroscience. doi:10.1523/JNEUROSCI.0535-19.2019
Abstract
Intracortical dynamics underlying repetitive stimulation predicts changes in network connectivity
Targeted stimulation can be used to modulate the activity of brain networks. Previously we demonstrated that direct electrical stimulation produces predictable post-stimulation changes in brain excitability. However, understanding the neural dynamics during stimulation and its relationship to post-stimulation effects is limited but critical for treatment optimization. Here, we applied 10Hz direct electrical stimulation across several cortical regions in 14 human subjects (6 males) implanted with intracranial electrodes for seizure monitoring. The stimulation train was characterized by a consistent increase in high gamma (70-170Hz) power. Immediately post-train, low-frequency (1-8Hz) power increased, resulting in an evoked response that was highly correlated with the neural response during stimulation. Using two measures of network connectivity, cortico-cortical evoked potentials (indexing effective connectivity) and theta coherence (indexing functional connectivity), we found a stronger response to stimulation in regions that were highly connected to the stimulation site. In these regions, repeated cycles of stimulation trains and rest progressively altered the stimulation response. Finally, after just two minutes (∼10%) of repetitive stimulation, we were able to predict post-stimulation connectivity changes with high discriminability. Taken together, this work reveals a relationship between stimulation dynamics and post-stimulation connectivity changes in humans. Thus, measuring neural activity during stimulation can inform future plasticity-inducing protocols.
SIGNIFICANCE STATEMENT
Brain stimulation tools have the potential to revolutionize the treatment of neuropsychiatric disorders. Despite the widespread use of brain stimulation techniques such as transcranial magnetic stimulation, the therapeutic efficacy of these technologies remains suboptimal. This is in part due to a lack of understanding of the dynamic neural changes that occur during stimulation. In this study, we provide the first detailed characterization of neural activity during plasticity induction through intracranial electrode stimulation and recording in 14 medication-resistant seizure patients. These results fill a missing gap in our understanding of stimulation-induced plasticity in humans. In the longer term, these data will also guide our translational efforts toward non-invasive, personalized, closed-loop neuromodulation therapy for neurological and psychiatric disorders in humans.






