Summary: Researchers have improved retrograde virus tracing to better reconstruct neural circuits in rats and mice.
Source: Cold Spring Harbor Laboratory.
New technologies have been likened, famously, to magic. At first, even the few who understand how they work have a tendency to sit back and marvel. Soon, flaws and limitations are detected and the invention process begins again, resulting, almost always, in improvements.
Today a team led by Professor Adam Kepecs at Cold Spring Harbor Laboratory (CSHL) describes in the journal Neuron a technology that improves on one that only a decade ago seemed magical – but alas, does no longer. Both technologies have the aim of enabling neuroscientists to relate the connectivity of specific types of neurons to the functions they perform – for instance, while an animal is performing a behavioral task. The new technology is an important improvement, however, making new things possible.
Being able to scrutinize structure/function relationships in different areas of the brain has been a huge step forward in learning basic principles that enable the brains of mammals, including us, to make sense of the world. About a decade ago, a new method called retrograde viral tracing was introduced. The technique exploits the ability of certain viruses to hijack a neuron in the brain – entering it at a place adjacent to where it connects with other neurons and traveling backward, along its threadlike axon, to the main body of the cell, called the soma. Retrograde tracing has been a boon to the field because it has helped researchers draw links between the functions of neurons located in widely separated brain areas.
These retrograde viruses are “frankly bizarre, real oddballs,” says Kepecs, “but incredibly useful. At this point, however, our field has matured and we now need them to do things they can’t always do.”
The problem is simply put: retrograde viruses often work, but sometime don’t. A researcher trying to trace a long-distance circuit may run into the problem of “tropism,” which is the inability of the virus to infect certain types of neurons. Since tropism is common, negative results are hard to interpret: is there no connection (i.e., neural pathway), or did the retrograde virus simply fail to infect the target neuron?
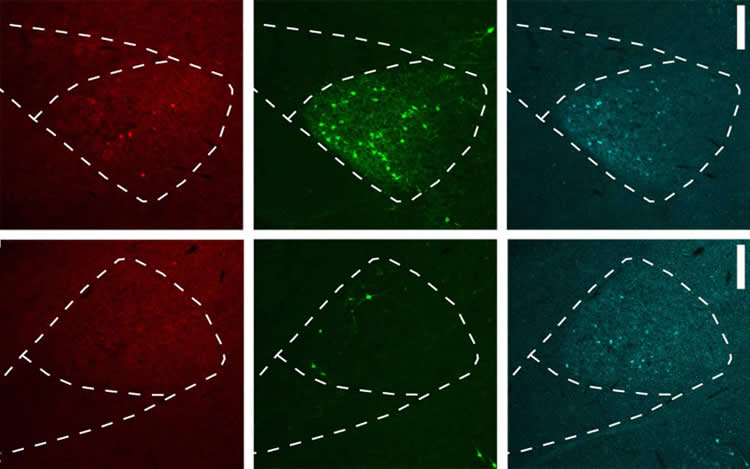
Kepecs’ team, led by Dr. Shujing Li, has come up with an improved version of retrograde tracing that eliminates the problem of tropism. The solution, called “receptor complementation,” centers on the concept of forcing the target cell to express a receptor that can interact with the virus and allow its entry. “It’s a little like we’re going around changing the locks on all the doors.” says Kepecs, “They’re still locked, but now we have all the keys.”
The team at CSHL successfully tested the method on a variety of neural circuits in both rats and mice, and showed that their method successfully reconstructed circuits that were lost to other methods. Finally, they engineered their viruses to express tools for controlling and recording neuronal activity – ensuring that the magic of retrograde tracing would continue for another day.
Funding: Funding provided by National Science Foundation (EAGER grant); National Institutes of Health.
Source: Peter Tarr – Cold Spring Harbor Laboratory
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Kepecs Lab, CSHL.
Original Research: Abstract for “A Viral Receptor Complementation Strategy to Overcome CAV-2 Tropism for Efficient Retrograde Targeting of Neurons” by Shu-Jing Li, Alexander Vaughan, James Fitzhugh Sturgill, and Adam Kepecs in Neuron. Published June 6 2018.
doi:10.1016/j.neuron.2018.05.028
[cbtabs][cbtab title=”MLA”]Cold Spring Harbor Laboratory “A Better Way to Trace Neural Pathways.” NeuroscienceNews. NeuroscienceNews, 6 June 2018.
<https://neurosciencenews.com/neural-pathway-tracing-9270/>.[/cbtab][cbtab title=”APA”]Cold Spring Harbor Laboratory (2018, June 6). A Better Way to Trace Neural Pathways. NeuroscienceNews. Retrieved June 6, 2018 from https://neurosciencenews.com/neural-pathway-tracing-9270/[/cbtab][cbtab title=”Chicago”]Cold Spring Harbor Laboratory “A Better Way to Trace Neural Pathways.” https://neurosciencenews.com/neural-pathway-tracing-9270/ (accessed June 6, 2018).[/cbtab][/cbtabs]
Abstract
A Viral Receptor Complementation Strategy to Overcome CAV-2 Tropism for Efficient Retrograde Targeting of Neurons
Highlights
•A receptor complementation strategy enables tropism-free retrograde viral delivery
•Enhanced CAV-2 retrograde spread in long-range projections in both mice and rats
•Vectors delivering CAR and different cargos for anatomical and functional studies
Summary
Retrogradely transported neurotropic viruses enable genetic access to neurons based on their long-range projections and have become indispensable tools for linking neural connectivity with function. A major limitation of viral techniques is that they rely on cell-type-specific molecules for uptake and transport. Consequently, viruses fail to infect variable subsets of neurons depending on the complement of surface receptors expressed (viral tropism). We report a receptor complementation strategy to overcome this by potentiating neurons for the infection of the virus of interest—in this case, canine adenovirus type-2 (CAV-2). We designed AAV vectors for expressing the coxsackievirus and adenovirus receptor (CAR) throughout candidate projection neurons. CAR expression greatly increased retrograde-labeling rates, which we demonstrate for several long-range projections, including some resistant to other retrograde-labeling techniques. Our results demonstrate a receptor complementation strategy to abrogate endogenous viral tropism and thereby facilitate efficient retrograde targeting for functional analysis of neural circuits.