Summary: Researchers have performed an MRI study of people with a common form of genetic autism. They discovered those with abnormalities on 16p11.2 had structural abnormalities with the corpus callosum and white matter volume.
Source: RSNA.
In the first major study of its kind, researchers using MRI have identified structural abnormalities in the brains of people with one of the most common genetic causes of autism, according to a new study published online in the journal Radiology. The abnormalities visible on brain images corresponded to cognitive and behavioral impairments in the study group, suggesting a future role for imaging in identifying people with autism who are in most urgent need of intervention.
Autism spectrum disorders are a group of developmental problems, affecting more than 3.5 million people in the U.S., according to the Centers for Disease Control and Prevention. Symptoms typically appear early in life and frequently include communication problems and repetitive behaviors. Many people with autism have abnormalities at a specific site on the 16th chromosome known as 16p11.2. Deletion or duplication of a small piece of chromosome at this site is one of the most common genetic causes of autism spectrum disorder.
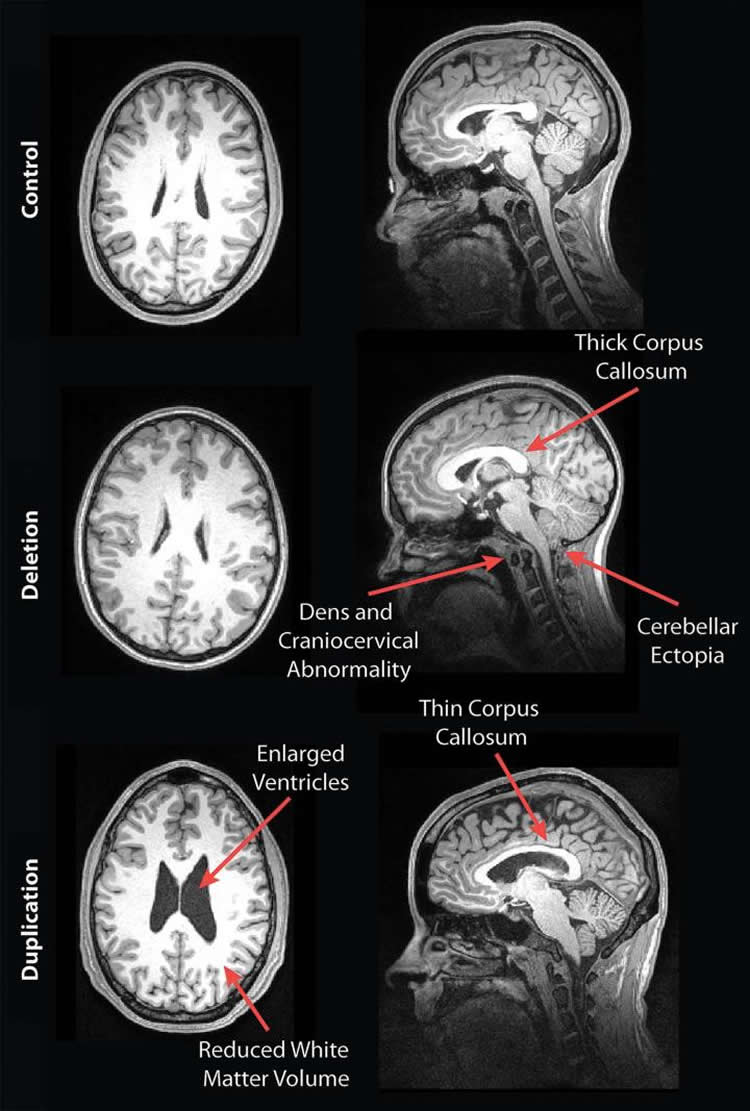
“People with deletions tend to have brain overgrowth, developmental delays and a higher risk of obesity,” said study author Julia P. Owen, Ph.D., a brain researcher at the University of Washington in Seattle, who was at the University of California in San Francisco (UCSF) during the study. “Those with duplications are born with smaller brains and tend to have lower body weight and developmental delays.”
The researchers at UCSF and four other sites performed structural MRI exams on 79 deletion carriers, ranging in age from 1 to 48, and 79 duplication carriers, ages 1 to 63, along with 64 unaffected family members and 109 participants in a control group.
The participants completed a battery of cognitive and behavioral tests, and neuroradiologists reviewed the brain images for development-related abnormalities. The results showed some striking differences in the brain structures of deletion and duplication carriers compared with non-carriers. For instance, the corpus callosum, the fiber bundle that connects the left and right sides of the brain, was abnormally shaped and thicker in the deletion carriers but thinner in the duplication carriers, compared to the control group and familial non-carriers.
Other stark differences were apparent. The deletion carriers displayed features of brain overgrowth, including the extension of the cerebellum, the bottom back part of the brain, toward the spinal cord. The duplication carriers showed characteristics of brain undergrowth, such as decreased white matter volume and larger ventricles — the cavities in the brain filled with cerebrospinal fluid.
When the researchers compared cognitive assessments to imaging findings, they found that the presence of any imaging feature associated with the deletion carriers — such as a thicker corpus callosum — indicated worse daily living, communication and social skills, compared to deletion carriers without any radiological abnormalities. For the duplication carriers, the presence of decreased white matter and corpus callosal volume and increased ventricle size was associated with decreased full-scale and verbal IQ scores, compared to duplication carriers without those findings.
Key to the study’s strength was its access to a large, diverse group of 16p11.2 deletion and duplication carriers, according to senior author Elliott Sherr, M.D., Ph.D., head of the Brain Development Research Program at UCSF.
“Often studies like this focus on high-functioning individuals, but this was an ‘all-comers’ group,” he said. “We didn’t do mathematical algorithms but rather used the trained eyes of neuroradiologists to evaluate the scans of a full range of individuals. When you look at a broad range of people like this, from developmentally normal to more significantly challenged, you’re better able to find these correlations.”
“Brain MR Imaging Findings and Associated Outcomes in Carriers of the Reciprocal Copy Number Variation at 16p11.2.” Collaborating with Drs. Owen and Sherr were Polina Bukshpun, B.A., Nicholas Pojman, B.S., Tony Thieu, M.S., Qixuan Chen, Ph.D., Jihui Lee, M.S., Debra D’Angelo, M.S., Orit A. Glenn, M.D., Jill V. Hunter, M.D., Jeffrey I. Berman, Ph.D., Timothy P. Roberts, Ph.D., Randy Buckner, Ph.D., Srikantan S. Nagarajan, Ph.D., and Pratik Mukherjee, M.D., Ph.D.
Funding: The study was supported by the Simons Variation in Individuals Project, or Simons VIP (sponsored by the Simons Foundation).
Source: Linda Brooks – RSNA
Image Source: NeuroscienceNews.com image is credited to the researchers/RSNA.
Original Source: Full open access research for “Brain MR Imaging Findings and Associated Outcomes in Carriers of the Reciprocal Copy Number Variation at 16p11.2” by Julia P. Owen, Polina Bukshpun, Nicholas Pojman, Tony Thieu, Qixuan Chen, Jihui Lee, Debra D’Angelo, Orit A. Glenn, Jill V. Hunter, Jeffrey I. Berman, Timothy P. Roberts, Randy Buckner, Srikantan S. Nagarajan, Pratik Mukherjee, and Elliott H. Sherr in Radiology/em>. Published online August 8 2017 doi:10.1148/radiol.2017162934
[cbtabs][cbtab title=”MLA”]RSNA “MRI Reveals Striking Brain Differences in People With Genetics Autism.” NeuroscienceNews. NeuroscienceNews, 8 August 2017.
<mri-genetic-autism-7264/>.[/cbtab][cbtab title=”APA”]RSNA (2017, August 8). MRI Reveals Striking Brain Differences in People With Genetics Autism. NeuroscienceNew. Retrieved August 8, 2017 from mri-genetic-autism-7264/[/cbtab][cbtab title=”Chicago”]RSNA “MRI Reveals Striking Brain Differences in People With Genetics Autism.” mri-genetic-autism-7264/ (accessed August 8, 2017).[/cbtab][/cbtabs]
Abstract
Brain MR Imaging Findings and Associated Outcomes in Carriers of the Reciprocal Copy Number Variation at 16p11.2
Autism spectrum disorder (ASD) affects one in 68 children and presents a major public health concern (1). Determining an association between neuroanatomical deviations and cognitive and behavioral sequelae in ASD has been difficult because of its heterogeneous etiologic causes. By focusing on individuals who share a causative genetic event, such as a single copy number variant (CNV) linked to ASD phenotypes, understanding of the linkage between brain and behavior in ASD could be improved. Imaging studies of genetically homogeneous cohorts linked to ASD reveal consistent alterations in cortical white matter and in the morphologic structure of the corpus callosum, among other anatomic abnormalities (2–9).
A recurrent and reciprocal 593-kb deletion and duplication CNV pair at the 16p11.2 chromosome is associated with global developmental delay, intellectual disability, and schizophrenia and is perhaps the most common CNV associated with ASD (10–16). Approximately 0.6% of individuals with ASD have a 16p11.2 CNV (13). Early case studies of patients also suggested anatomic abnormalities, such as syringomyelia, Chiari I malformation, and ventriculomegaly (17,18). These preliminary studies provide a rationale for a detailed large-scale study of individuals with 16p11.2 CNVs to obtain magnetic resonance (MR) imaging data independent of clinical symptoms and to then directly assess associations between neuroanatomical changes and clinical outcomes.
The Simons Variation in Individuals Project (VIP) (19) examined a large cohort of individuals with a range of clinical phenotypes who all had a 16p11.2 CNV. Unaffected family members and population control participants were included for comparison. Several articles were published from the extensive neuropsychologic and imaging data collected as part of the Simons VIP (13,20–25). The purpose of our study was to identify the salient neuroradiologic findings in a large cohort of 16p11.2 deletion and duplication carriers by using well-established clinical radiologic assessments from over 300 participants and to assess how these features are associated with behavioral and cognitive outcomes. Our central study hypotheses were that carriers of deletions and duplications at 16p11.2 (referred to here as deletion carriers and duplication carriers, respectively) would differ in the frequency of radiologic findings compared with both familial noncarriers and population control participants, and the presence of neuroradiologic abnormalities in the carriers would be associated with cognitive or behavioral deficits. Unsupervised machine learning extracted a nonoverlapping constellation of features associated with the deletion and duplication carriers, which provided imaging features that could constitute a radiologic syndrome for 16p11.2 deletion and duplication carriers.
“Brain MR Imaging Findings and Associated Outcomes in Carriers of the Reciprocal Copy Number Variation at 16p11.2” by Julia P. Owen, Polina Bukshpun, Nicholas Pojman, Tony Thieu, Qixuan Chen, Jihui Lee, Debra D’Angelo, Orit A. Glenn, Jill V. Hunter, Jeffrey I. Berman, Timothy P. Roberts, Randy Buckner, Srikantan S. Nagarajan, Pratik Mukherjee, and Elliott H. Sherr in Radiology/em>. Published online August 8 2017 doi:10.1148/radiol.2017162934






