Summary: Astrocytes enhance healing following a brain hemorrhage by transferring their mitochondria to damaged neurons.
Source: SfN
After a brain hemorrhage, neural support cells called astrocytes enhance healing by transferring their mitochondria to damaged neurons.
The healthy mitochondria stimulate the production of a free radical-fighting enzyme, according to new research published in Journal of Neuroscience.
An artery in the brain bursts. Blood rushes into the tissue, inducing free radicals that cause even more damage.
The hemorrhage damages mitochondria, the site of energy production in cells. Astrocytes transfer their mitochondria to damaged neurons after a hemorrhage.
These healthy mitochondria contain a “healing” peptide called humanin and an enzyme called manganese superoxide dismutase (Mn-SOD) that help neutralize free radicals.
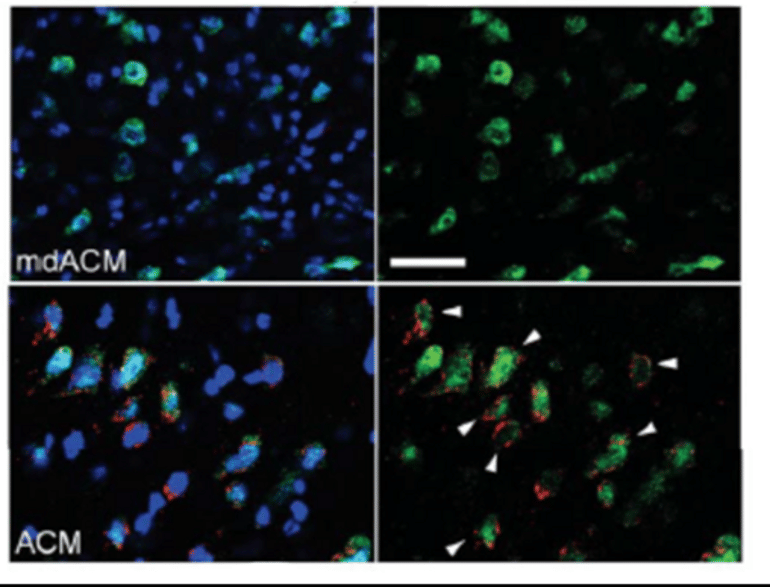
Tashiro et al. injected mice with healthy mitochondria after a hemorrhage. The hemorrhage reduced levels of Mn-SOD in the mice brain and increased the number of free radicals.
Using molecular tags, the researchers found that the rodents’ neurons took up the mitochondria from the bloodstream.
The mice who received the treatment showed improved neurological recovery, but the benefits decreased if the mice received mitochondria without the Mn-SOD enzyme.
These results reveal mitochondria can transfer between brain cells to improve health and aide recovery.
About this neuroscience research news
Author: Calli McMurray
Source: SfN
Contact: Calli McMurray – SfN
Image: The image is credited to Tashiro et al., JNeurosci 2022
Original Research: Closed access.
“Transplantation of astrocytic mitochondria modulates neuronal antioxidant defense and neuroplasticity and promotes functional recovery after intracerebral hemorrhage” by Tashiro et al. Journal of Neuroscience
Abstract
Transplantation of astrocytic mitochondria modulates neuronal antioxidant defense and neuroplasticity and promotes functional recovery after intracerebral hemorrhage
Astrocytes release functional mitochondria (Mt) that play regulatory and pro-survival functions upon entering adjacent cells. We recently demonstrated that these released Mt could enter microglia to promote their reparative/pro-phagocytic phenotype that assists in hematoma cleanup and neurological recovery after intracerebral hemorrhage (ICH).
However, a relevance of astrocytic Mt transfer into neurons in protecting brain after ICH is unclear. Here, we found that ICH causes a robust increase in superoxide generation and elevated oxidative damage that coincides with loss of the mitochondrial enzyme manganese superoxide dismutase (Mn-SOD).
The damaging effect of ICH was reversed by intravenous transplantation of astrocytic Mt that upon entering the brain (and neurons), restored Mn-SOD levels and reduced neurological deficits in male mice subjected to ICH. Using an in vitro ICH-like injury model in cultured neurons, we established that astrocytic Mt upon entering neurons prevented reactive oxygen species-induced oxidative stress and neuronal death by restoring neuronal Mn-SOD levels, while at the same time promoted neurite extension and upregulation of synaptogenesis-related gene expression.
Furthermore, we found that Mt genome-encoded small peptide humanin (HN) that is normally abundant in Mt, could simulate Mt-transfer effect on neuronal Mn-SOD expression, oxidative stress, and neuroplasticity under ICH-like injury.
This study demonstrates that adoptive astrocytic Mt transfer enhances neuronal Mn-SOD-mediated anti-oxidative defense and neuroplasticity in the brain, which potentiate functional recovery following ICH.






