Summary: Researchers have identified a novel type of cell death associated with Huntington’s disease.
Source: Tokyo Medical and Dental University.
Researchers centered at Tokyo Medical and Dental University (TMDU) identify novel type of cell death in Huntington’s disease that may uncover new treatments.
In Huntington’s disease (HD), the huntingtin gene is mutated, causing progressive neuronal death. This leads to defects in movement, behavior, and cognitive ability. Apoptosis, autophagy, and necrosis are the three main types of cell death, but researchers have not yet been able to determine what type of cell death causes neurodegeneration in the brain of HD patients.
In a new study, Tokyo Medical and Dental University-led researchers examined the nature of cell death in HD using newly developed imaging techniques. The effects of mutant huntingtin in neuronal cells were visualized by live cell imaging. With this approach, the authors identified a novel type of cell death associated with mutant huntingtin, which they called ballooning cell death (BCD). These cells gradually expanded like a balloon, until they ruptured.
To characterize the specific nature of BCD, the authors examined different cellular organelles by live cell imaging. “The endoplasmic reticulum was the main origin of ballooning,” study first author Ying Mao explains. “Rupture of the endoplasmic reticulum into the cytosol was followed by gradual cell body ballooning, nuclear shrinkage, and cell rupture.”
The authors observed the same phenomena in vivo using two-photon endoplasmic reticulum imaging in a HD mouse model.
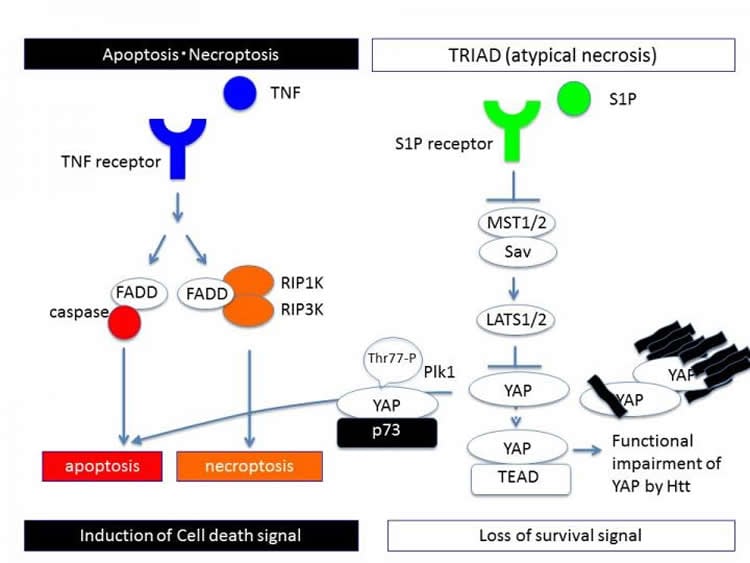
Pharmacological inhibitors and genetic interventions showed that BCD was not like apoptosis or autophagy. “We noticed multiple similarities between BCD and a unique form of necrosis called TRIAD, which is caused by inhibition of RNA polymerase II in neurons,” corresponding author Hitoshi Okazawa explains. “Based on our existing knowledge of how TRIAD is regulated, we were able to show that BCD is mediated by impaired TEAD/YAP transcription.”
These revelations provided the opportunity to test potential therapeutic targets for HD. The researchers introduced S1P and up-regulated TEAD/YAP transcription in HD mice. This stabilized endoplasmic reticulum and completely stopped the decline of motor function, suggesting that targeting TEAD/YAP-dependent necrosis may lead to development of effective therapies for HD.
Funding: The work was supported by Ministry of Education, Culture, Sports, Science and Technology, JAPAN.
Source: Hitoshi Okazawa – Tokyo Medical and Dental University
Image Source: This NeuroscienceNews.com image is credited to Department of Neuropathology,Medical Research Institute.
Original Research: Abstract for “Targeting TEAD/YAP-transcription-dependent necrosis, TRIAD, ameliorates Huntington’s disease pathology” by Ying Mao, Xigui Chen, Min Xu, Kyota Fujita, Kazumi Motoki, Toshikazu Sasabe, Hidenori Homma, Miho Murata, Kazuhiko Tagawa, Takuya Tamura, Julia Kaye, Steven Finkbeiner, Giovanni Blandino, Marius Sudol, and Hitoshi Okazawa in Human Molecular Genetics. Published online September 6 2016 doi:10.1093/hmg/ddw303
[cbtabs][cbtab title=”MLA”]Tokyo Medical and Dental University. “Novel Type of Cell Death in Huntington’s Disease May Lead to Effective New Therapies.” NeuroscienceNews. NeuroscienceNews, 22 November 2016.
<https://neurosciencenews.com/huntingtons-cell-death-5583/>.[/cbtab][cbtab title=”APA”]Tokyo Medical and Dental University. (2016, November 22). Novel Type of Cell Death in Huntington’s Disease May Lead to Effective New Therapies. NeuroscienceNews. Retrieved November 22, 2016 from https://neurosciencenews.com/huntingtons-cell-death-5583/[/cbtab][cbtab title=”Chicago”]Tokyo Medical and Dental University. “Novel Type of Cell Death in Huntington’s Disease May Lead to Effective New Therapies.” https://neurosciencenews.com/huntingtons-cell-death-5583/ (accessed November 22, 2016).[/cbtab][/cbtabs]
Abstract
Targeting TEAD/YAP-transcription-dependent necrosis, TRIAD, ameliorates Huntington’s disease pathology
Neuronal cell death in neurodegenerative diseases is not fully understood. Here we report that mutant huntingtin (Htt), a causative gene product of Huntington’s diseases (HD) selectively induces a new form of necrotic cell death, in which endoplasmic reticulum (ER) enlarges and cell body asymmetrically balloons and finally ruptures. Pharmacological and genetic analyses revealed that the necrotic cell death is distinct from the RIP1/3 pathway-dependent necroptosis, but mediated by functional deficiency of TEAD/YAP-dependent transcription. In addition, we revealed that a cell cycle regulator, Plk1, switches the balance between TEAD/YAP-dependent necrosis and p73/YAP-dependent apoptosis by shifting the interaction partner of YAP from TEAD to p73 through YAP phosphorylation at Thr77. In vivo ER imaging with two-photon microscopy detects similar ER enlargement, and viral vector-mediated delivery of YAP as well as chemical inhibitors of Hippo pathway such as S1P recover the ER instability and necrosis in HD model mice. Intriguingly S1P completely stops the decline of motor function of HD model mice even after the onset of symptom. Collectively, we suggest approaches targeting the signaling pathway of TEAD/YAP-transcription-dependent necrosis (TRIAD) could lead to a therapeutic development against HD.
“Targeting TEAD/YAP-transcription-dependent necrosis, TRIAD, ameliorates Huntington’s disease pathology” by Ying Mao, Xigui Chen, Min Xu, Kyota Fujita, Kazumi Motoki, Toshikazu Sasabe, Hidenori Homma, Miho Murata, Kazuhiko Tagawa, Takuya Tamura, Julia Kaye, Steven Finkbeiner, Giovanni Blandino, Marius Sudol, and Hitoshi Okazawa in Human Molecular Genetics. Published online September 6 2016 doi:10.1093/hmg/ddw303