Summary: Researchers from King’s College London have identified a single protein molecule that may be responsible for chronic nerve pain in diabetes.
Source: King’s College London.
A new King’s College London study reveals the molecular basis of chronic nerve pain in diabetes. The findings in mice, published today in Science Translational Medicine, could one day lead to treatments which target the source of the pain.
Around one in four people with diabetes develop a chronic pain condition induced by nerve damage, called painful diabetic neuropathy (PDN), due to high blood sugar. Symptoms include prickling and tingling sensations as well as sharp, shooting pains and extreme sensitivity to touch in the feet and hands, which can spread upward into the legs and arms. The pain can significantly impair mobility, which in turn exacerbates obesity and worsens type 2 diabetes in a self-perpetuating cycle.
Diabetic pain is very difficult to treat and the molecular causes are poorly understood. This new King’s College London study provides the first evidence that a single protein molecule – HCN2 – can by itself be responsible for a complex sensation such as diabetic pain.
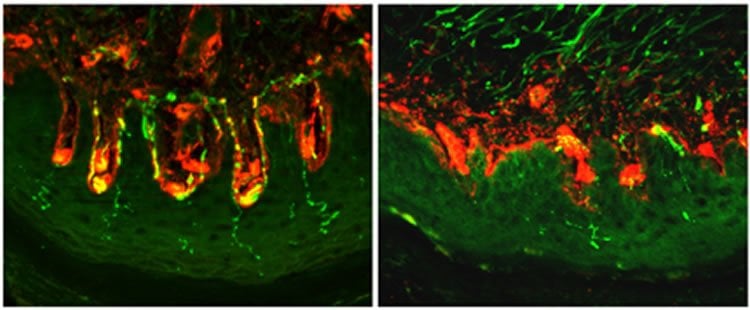
The researchers used mouse models of diabetes to show that over-activity of HCN2, which initiates electrical signals in pain-sensitive nerve fibres, results in a sensation of pain. They also found that blocking the activity of HCN2, or removing it completely from pain-sensitive nerve fibres, stopped the sensation of pain entirely.
Professor Peter McNaughton, senior author of the study, from the Wolfson Centre for Age-Related Diseases at King’s College London, said: ‘The inexorable rise of obesity worldwide, in both rich and poorer countries, has brought a related increase in type 2 diabetes. As many as one in four diabetics suffer from nerve pain, yet there are currently no effective treatments and people therefore typically must resign themselves to a life of continuous suffering.
‘Our study reveals the molecular mechanism driving diabetic pain in mice, which we hope will inform future treatments in people with diabetes.’
Dr Christoforos Tsantoulas, first author of the study, also from the Wolfson Centre for Age-Related Diseases at King’s College London, said: ‘At present we do not have selective drugs which can suppress the activity of HCN2 without affecting other bodily functions, such as the regulation of heart rate. This research provides a stimulus for the development of targeted pain drugs that can block HCN2 without affecting the activity of other molecules.’
Funding: The main funder of this study was the Medical Research Council (MRC).
Neuroscience News would like to thank Chris Tsantoulas, PhD for submitting this article directly to us.
Source: Jack Stonebridge – King’s College London
Image Source: NeuroscienceNews.com image is adapted from the King’s College London news release.
Original Research: Abstract for “Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy” by Christoforos Tsantoulas, Sergio Laínez, Sara Wong, Ishita Mehta, Bruno Vilar and Peter A. McNaughton in Science Translational Medicine. Published online September 26 2017 doi:10.1126/scitransimed.aam6072
[cbtabs][cbtab title=”MLA”]King’s College London “Researchers Uncover Source of Diabetic Pain.” NeuroscienceNews. NeuroscienceNews, 29 September 2017.
<https://neurosciencenews.com/hcn2-diabetic-pain-7615/>.[/cbtab][cbtab title=”APA”]King’s College London (2017, September 29). Researchers Uncover Source of Diabetic Pain. NeuroscienceNews. Retrieved September 29, 2017 from https://neurosciencenews.com/hcn2-diabetic-pain-7615/[/cbtab][cbtab title=”Chicago”]King’s College London “Researchers Uncover Source of Diabetic Pain.” https://neurosciencenews.com/hcn2-diabetic-pain-7615/ (accessed September 29, 2017).[/cbtab][/cbtabs]
Abstract
Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy
Diabetic patients frequently suffer from continuous pain that is poorly treated by currently available analgesics. We used mouse models of type 1 and type 2 diabetes to investigate a possible role for the hyperpolarization-activated cyclic nucleotide–gated 2 (HCN2) ion channels as drivers of diabetic pain. Blocking or genetically deleting HCN2 channels in small nociceptive neurons suppressed diabetes-associated mechanical allodynia and prevented neuronal activation of second-order neurons in the spinal cord in mice. In addition, we found that intracellular cyclic adenosine monophosphate (cAMP), a positive HCN2 modulator, is increased in somatosensory neurons in an animal model of painful diabetes. We propose that the increased intracellular cAMP drives diabetes-associated pain by facilitating HCN2 activation and consequently promoting repetitive firing in primary nociceptive nerve fibers. Our results suggest that HCN2 may be an analgesic target in the treatment of painful diabetic neuropathy.
“Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy” by Christoforos Tsantoulas, Sergio Laínez, Sara Wong, Ishita Mehta, Bruno Vilar and Peter A. McNaughton in Science Translational Medicine. Published online September 26 2017 doi:10.1126/scitransimed.aam6072