Summary: Targeting a protein called neuroligin 3 helps stop the growth of high grade gliomas in mice, a new study reports.
Source: NIH/NINDS.
New research conducted in mice provides evidence that highly lethal brain tumors, called high-grade gliomas, stop growing when deprived of a specific molecule naturally produced when brain cells fire. The experiments, led by a group of scientists from Stanford University, Palo Alto, California, suggest that targeting a protein called neuroligin-3 may prove beneficial in patients with these diseases. The work was published in Nature and supported by the National Institute of Neurological Disorders and Stroke (NINDS), part of the National Institutes of Health.
“This study transforms our understanding of how neurons influence the growth of gliomas, and opens a new door for potential treatments,” said NINDS Program Director Jane Fountain, Ph.D.
High-grade gliomas cause more deaths than any other form of brain cancer, partly due to the extreme difficulty surgeons have in removing all of the tumor cells. This leaves clinicians dependent on traditional chemotherapy and radiation treatments that have limited success. Depending on the specific subtype of tumor, more than three-quarters of patients die within five years, and for the most common childhood glioma that number exceeds 99 percent.
“Treating these tumors is very difficult, in part because we don’t fully understand what drives them,” said senior author Michelle Monje, M.D., Ph.D, an assistant professor of neurology at Stanford.
In a 2015 paper published in Cell, Dr. Monje’s team identified several chemicals released by brain cells in response to neural activity that cause high-grade gliomas to grow. One of them was neuroligin-3, a protein that helps neurons communicate.
In the current study, the researchers extracted tumor cells from patients with several varieties of high-grade gliomas and inserted them into the brains of two breeds of mice, one normal and one lacking the gene that produces neuroligin-3. In the latter, none of the tumors grew substantially for four and a half months and roughly half remained stagnant after six months, whereas tumors grew markedly in the mice with an intact neuroligin-3 gene. Further experiments suggested neuroligin-3 triggers a series of chemical reactions that stimulates multiple signaling pathways involved in glioma growth, causing the tumors to expand.
“We knew neuroligin-3 was one of many secreted factors that can promote tumor growth, but we didn’t expect it to be so essential,” said Dr. Monje. “I think that speaks to something fundamental that neuroligin-3 is doing in high-grade glioma that we need to better understand.”
The team also discovered that neuroligin-3 release is triggered when active neurons secrete a protein called ADAM10, which causes neuroligin-3 to detach from the surface of cells. Stopping neurons from firing prevented the release of both ADAM10 and neuroligin-3, and genetically deleting ADAM10 from neurons in mice reduced neuroligin-3 release. In addition, the team found that a group of brain cells called oligodendrocyte precursor cells can release neuroligin-3, suggesting those cells may play a role in accelerating glioma growth.
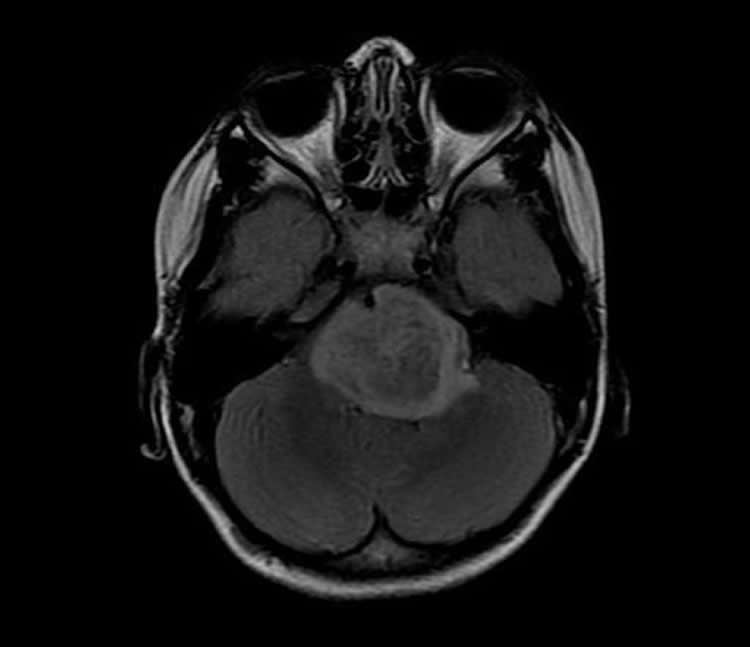
Finally, Dr. Monje’s group showed that blocking ADAM10 activity with a drug designed to treat other types of cancers dramatically reduced the growth of two types of gliomas implanted into the brains of mice.
“That’s really exciting because it may be that we can use ADAM10 inhibition as a complement to the therapeutic strategy we are already using,” said Dr. Monje.
In addition to working towards a better understanding of why gliomas are so dependent on neuroligin-3 for growth, Dr. Monje’s team is now hoping to initiate a clinical trial using an ADAM10 inhibitor in human glioma patients. She is also interested in how neuroligin-3 binds to glioma cells, research that could lead to a method of slowing glioma growth by preventing this interaction. Although treatments targeting ADAM10 and neuroligin-3 might extend patients’ lifespans, they would not kill the tumors, making additional treatments necessary to cure the disease.
“I am a pediatric neuro-oncologist — I take care of patients with these tumors and I have no effective therapy for them,” Dr. Monje said. “There is an urgent need to find a better therapy for gliomas, and I think that we are now pursuing an avenue of research that holds great promise.”
Funding: The study was funded by NINDS (NS092597), the National Cancer Institute (CA200273, CA168504, CA165962, CA220057), and the intramural program of the National Center for Advancing Translational Sciences (NCATS), as well as the V Foundation; the Liwei Wang Research Fund; the Department of Defense; the McKenna Claire Foundation; Alex’s Lemonade Stand Foundation; The Cure Starts Now Foundation and DIPG Collaborative; the Lyla Nsouli Foundation; Unravel Pediatric Cancer; the California Institute for Regenerative Medicine; the Childhood Brain Tumor Foundation; the Matthew Larson Foundation; the Joey Fabus Childhood Cancer Foundation; the Wayland Villars DIPG Foundation; the Connor Johnson, Zoey Ganesh, and Declan Gloster Memorial Funds; the N8 Foundation, the Virgina and D.K. Ludwig Fund for Cancer Research, the Stanford Child Health Research Institute Anne T. and Robert M. Bass Endowed Faculty Scholarship in Pediatric Cancer and Blood Diseases, and the Breast Cancer Research Foundation.
Source: Brandon Levy – NIH/NINDS
Image Source: NeuroscienceNews.com image is credited to Michelle Monje, M.D., Ph.D., Stanford University.
Original Research: Abstract for “Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma” by Humsa S. Venkatesh, Lydia T. Tam, Pamelyn J. Woo, James Lennon, Surya Nagaraja, Shawn M. Gillespie, Jing Ni, Damien Y. Duveau, Patrick J. Morris, Jean J. Zhao, Craig J. Thomas & Michelle Monje in Nature. Published online September 20 2017 doi:10.1038/nature24014
[cbtabs][cbtab title=”MLA”]NIH/NINDS “Hitting the Breaks on Lethal Brain Cancers.” NeuroscienceNews. NeuroscienceNews, 22 September 2017.
<https://neurosciencenews.com/glioma-neurology-7546/>.[/cbtab][cbtab title=”APA”]NIH/NINDS (2017, September 22). Hitting the Breaks on Lethal Brain Cancers. NeuroscienceNew. Retrieved September 22, 2017 from https://neurosciencenews.com/glioma-neurology-7546/[/cbtab][cbtab title=”Chicago”]NIH/NINDS “Hitting the Breaks on Lethal Brain Cancers.” https://neurosciencenews.com/glioma-neurology-7546/ (accessed September 22, 2017).[/cbtab][/cbtabs]
Abstract
Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma
High-grade gliomas (HGG) are a devastating group of cancers, and represent the leading cause of brain tumour-related death in both children and adults. Therapies aimed at mechanisms intrinsic to glioma cells have translated to only limited success; effective therapeutic strategies will need also to target elements of the tumour microenvironment that promote glioma progression. Neuronal activity promotes the growth of a range of molecularly and clinically distinct HGG types, including adult and paediatric glioblastoma (GBM), anaplastic oligodendroglioma, and diffuse intrinsic pontine glioma (DIPG)1. An important mechanism that mediates this neural regulation of brain cancer is activity-dependent cleavage and secretion of the synaptic adhesion molecule neuroligin-3 (NLGN3), which promotes glioma proliferation through the PI3K–mTOR pathway1. However, the necessity of NLGN3 for glioma growth, the proteolytic mechanism of NLGN3 secretion, and the further molecular consequences of NLGN3 secretion in glioma cells remain unknown. Here we show that HGG growth depends on microenvironmental NLGN3, identify signalling cascades downstream of NLGN3 binding in glioma, and determine a therapeutically targetable mechanism of secretion. Patient-derived orthotopic xenografts of paediatric GBM, DIPG and adult GBM fail to grow in Nlgn3 knockout mice. NLGN3 stimulates several oncogenic pathways, such as early focal adhesion kinase activation upstream of PI3K–mTOR, and induces transcriptional changes that include upregulation of several synapse-related genes in glioma cells. NLGN3 is cleaved from both neurons and oligodendrocyte precursor cells via the ADAM10 sheddase. ADAM10 inhibitors prevent the release of NLGN3 into the tumour microenvironment and robustly block HGG xenograft growth. This work defines a promising strategy for targeting NLGN3 secretion, which could prove transformative for HGG therapy.
“Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma” by Humsa S. Venkatesh, Lydia T. Tam, Pamelyn J. Woo, James Lennon, Surya Nagaraja, Shawn M. Gillespie, Jing Ni, Damien Y. Duveau, Patrick J. Morris, Jean J. Zhao, Craig J. Thomas & Michelle Monje in Nature. Published online September 20 2017 doi:10.1038/nature24014






