Summary: The direct phosphorylation of KCNQ2 by ERK occurs through the activation of the dopamine signaling pathway in the nucleus accumbens of mice.
Source: Fujita Health University
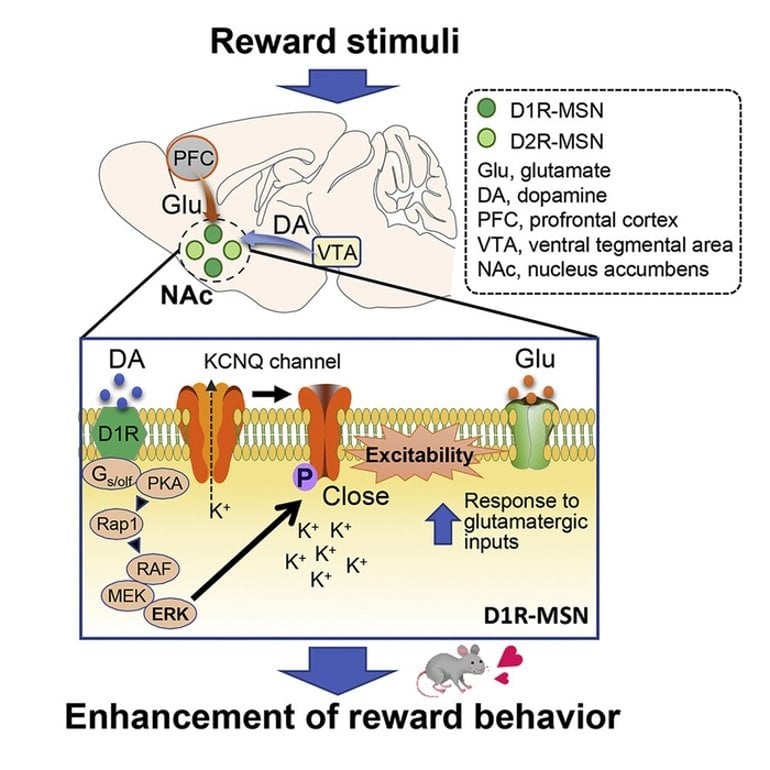
One part of the basal ganglia, known as the nucleus accumbens (NAc), is composed of medium spiny neurons (MSNs). The MSNs, in which the dopamine D1 receptor (D1R) and dopamine D2 receptor (D2R) express, are controlled by neurotransmitters—glutamate and dopamine.
Dopamine is an organic chemical that regulates memory and learning, motor functions, and emotional behavior for motivation and reward.
On increasing the excitability of D1R-MSNs, dopamine promotes glutamate-induced responses, leading to a reward behavior, i.e., feelings of accomplishment and pleasure associated with completion of certain tasks.
It is known that the molecular mechanism underlying this reward behavior involves a signaling cascade that increases the “phosphorylation” (the attachment of a phosphate group) of protein kinase A (PKA) substrates.
Subsequently, the extracellular signal-regulated kinase (ERK), such as a downstream effector of PKA, modulates the excitability of D1R MSNs and in turn, the reward behavior.
Recently, a team of researchers, including Assistant Professor Daisuke Tsuboi and Professor Kozo Kaibuchi of the Fujita Health University, and Dr. Nambu Atsushi and Dr. Hiromi Sano of the National Institute of Physiological Sciences in Japan, identified an ERK phospsho-substrate known as potassium voltage-gated channel subfamily Q member 2 (KCNQ2). Although certain studies have highlighted the role of KCNQ2 channels in MSN excitability, the details of the mechanism are still unclear.
Now, Dr. Tsuboi and his team have investigated the mechanism through which ERK regulates KCNQ2 activity and affects dopamine signaling.
“Dysfunctional dopamine signaling is implicated in several neuropsychological disorders, including Parkinson’s disease, drug addiction, and schizophrenia. Therefore, understanding the underlying pathway is crucial for treating several psychiatric diseases,” Dr. Tsuboi remarks while discussing the motivation behind the study, which was published in Volume 40, Issue 10 of Cell Reports on September 6, 2022.
The team conducted experiments and mice and observed that the activation of D1R receptors with an agonist inhibited KCNQ-dependent currents and led to increased D1R-MSN firing rates in NAc slices of mice brains. Interestingly, MSN firing was halted on inhibiting ERK, suggesting its involvement in the process.
Further analyses confirmed that the direct phosphorylation of KCNQ2 by ERK occurs through the activation of the dopamine signaling pathway in the NAc of mice.
Next, the team noted that ERK-mediated phosphorylation of KCNQ2 decreased its channel activity. Moreover, the conditional deletion of the Kcnq2 gene in the D1R-MSNs of mice reduced the inhibitory effect of dopamine on the KCNQ current, enhancing neuronal excitability and reward behavior.
These effects were rescued when the Kcnq2 gene was re-introduced, but not when its phospho-deficient mutant gene was introduced. This implies that dopamine signaling negatively regulates the currents via KNCQ phosphorylation.
Based on these findings, the team proposed the following mechanism: Dopamine acts on D1R-MSNs to activate the ERK pathway that induces the phosphorylation of KCNQ2. This decreases the channel activity of KCNQ, which increases cell excitability and promotes reward behavior in the presence of glutamate.
So, what are the long-term applications of these findings?
Dr. Kaibuchi explains, “Mood disorders such as depression show signs associated with dysfunction of the reward system circuits in the brain. Hence, drugs modulating the dopamine-KCNQ2 pathway can be potential therapeutic targets for drug addiction and mood disorders. This study has highlighted KCNQ2 as a potential target for such psychiatric diseases.”
Here’s hoping that these findings improve our understanding of the dopamine signaling pathway and its association with diverse emotional states!
About this dopamine research news
Author: Hisatsugu Koshimizu
Source: Fujita Health University
Contact: Hisatsugu Koshimizu – Fujita Health University
Image: The image is credited to Dr. Kozo Kaibuchi and Dr. Daisuke Tsuboi from Fujita Health University
Original Research: Open access.
“Dopamine drives neuronal excitability via KCNQ channel phosphorylation for reward behavior” by Daisuke Tsuboi et al. Cell Reports
Abstract
Dopamine drives neuronal excitability via KCNQ channel phosphorylation for reward behavior
Highlights
- Activation of D1R/PKA/ERK pathway increases phosphorylation of KCNQ2
- D1R signaling negatively regulates KCNQ currents through KCNQ2 phosphorylation
- KCNQ2 phosphorylation in D1R-MSNs increased the excitability of their neurons
- Conditional loss of KCNQ2 in D1R-MSNs facilitates cocaine-reward behavior
Summary
Dysfunctional dopamine signaling is implicated in various neuropsychological disorders. Previously, we reported that dopamine increases D1 receptor (D1R)-expressing medium spiny neuron (MSN) excitability and firing rates in the nucleus accumbens (NAc) via the PKA/Rap1/ERK pathway to promote reward behavior.
Here, the results show that the D1R agonist, SKF81297, inhibits KCNQ-mediated currents and increases D1R-MSN firing rates in murine NAc slices, which is abolished by ERK inhibition.
In vitro ERK phosphorylates KCNQ2 at Ser414 and Ser476; in vivo, KCNQ2 is phosphorylated downstream of dopamine signaling in NAc slices.
Conditional deletion of Kcnq2 in D1R-MSNs reduces the inhibitory effect of SKF81297 on KCNQ channel activity, while enhancing neuronal excitability and cocaine-induced reward behavior. These effects are restored by wild-type, but not phospho-deficient KCNQ2.
Hence, D1R-ERK signaling controls MSN excitability via KCNQ2 phosphorylation to regulate reward behavior, making KCNQ2 a potential therapeutical target for psychiatric diseases with a dysfunctional reward circuit.






