Summary: Medically induced loss of consciousness disrupts neuron population activity patterns by generating fewer discriminable network microstates, and fewer neuronal ensembles. Findings suggest local neuronal ensemble dynamics may contribute to the emergence of conscious states.
Source: Data Science Institute at Columbia
Medically-induced loss of consciousness (mLOC) during anesthesia is associated with a breakdown of brain connectivity at the anatomical macro scale – across cortical areas – yet what role brain microcircuitry plays in LOC remains unclear.
How does our brain generate our consciousness? And why do we lose it during anesthesia? Influential theories suggest that consciousness depends on the brain’s ability to discriminate between a specific sensory input and a large set of alternatives, akin to being able to choose one outcome among many.
Indeed, several studies in humans using fMRI have identified a rich set of resting states of cortical activity at the anatomical macro scale, that is, at the level of large brain areas. A research team led by Columbia University, however, hypothesized that a person’s ability to discriminate between a set of alternatives at any moment should be rooted in micro-patterns of activity, or microstates, at the level of local neuronal ensembles – the functional building blocks of neural circuits.
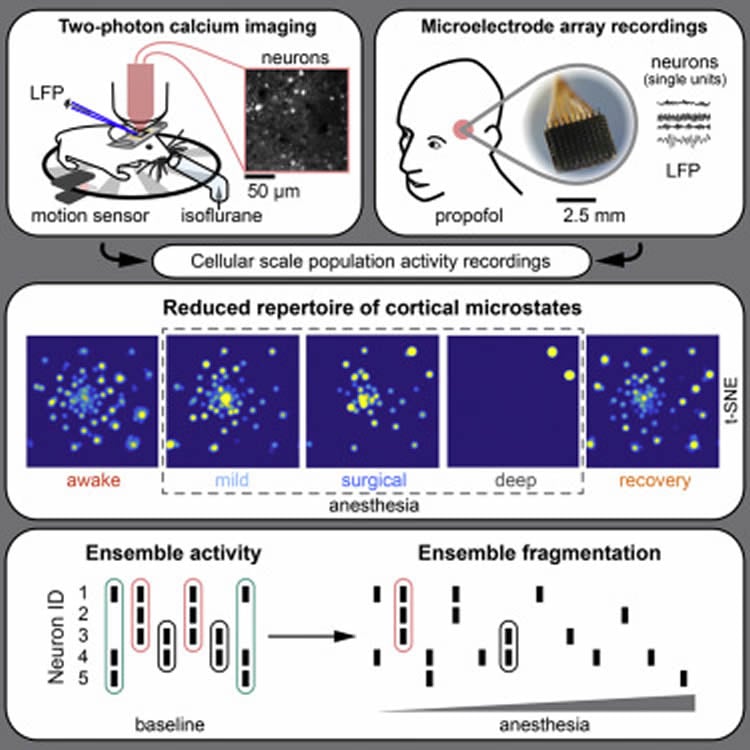
And in a first of its kind study, researchers from Columbia’s Rafael Yuste’s Laboratory used cellular resolution in vivo two-photon calcium imaging in mice to investigate changes in the local repertoire of neuronal microstates during anesthesia. The team found that anesthesia disrupts the number of neural patterns by reducing both network microstates and neuronal ensembles in the cortex, and confirmed their findings in microelectrode array recordings from two human subjects. Their results, published today in the journal, Cell Systems, indicate that the functional connectivity of the brain during mLOC breaks down across micro- and macro-anatomical scales.
“This study is important because it takes the question of the nature of consciousness to the neuronal circuit level,” said Rafael Yuste, director of the Yuste Lab and professor of Biological Sciences and Neuroscience at Columbia. “It provides an intellectual thread that could explain how alterations in the coordination of small groups of neurons, either by pathologies or anesthetics, could lead to alterations in consciousness,” added Yuste, who is also a member of Columbia’s Data Science Institute and an initiator of the BRAIN Initiative.
A popular notion maintains that while mLOC is associated with a macroscale breakdown of functional connectivity, local networks keep displaying dynamics similar to the awake state, yet in an isolated and insular manner, said Michael Wenzel, the lead author on the paper. “And while this suggests that LOC arises from a discoordination of neural activity across brain areas, we found that local network dynamics change dramatically during mLOC. Our results indicate that the loss of consciousness could arise from alterations in the local microcircuit, which would secondarily generate deficits in macroscale connectivity” added Wenzel, who at the time of study was an Associate Research Scientist in the Rafael Yuste Lab.
The study, “Reduced Repertoire of Cortical Microstates and Neuronal Ensembles in Medically Induced Loss of Consciousness,” offers a foundation for understanding how dynamics of local neuronal ensembles could contribute to the loss or emergence of conscious states. The authors noted that mechanistic studies on the role of local circuits in the loss or gain of consciousness remain challenging, though they hope their research adds to the basic science of the neural circuitry.
The other members of the research team are Shuting Han, Ph.D., Neuro Technology Center at Columbia (now at ETH Zurich), Elliot H. Smith, Ph.D., Department of Neurological Surgery at Columbia (now at the University of Utah); Erik Hoel, Ph.D., NeuroTechnology Center at Columbia (now at Tufts University); Bradley Greger, Ph.D., School of Biological and Health Systems Engineering, Arizona State University; Paul House, MD, Department of Neurosurgery, University of Utah; and Rafael Yuste, MD, Ph.D., the leader of the team and who also directs Columbia’s Neurotechnology Center.
Funding: The research was supported by The National Institute of Mental Health; the Neuroscience Education Institute; the U. S. Army Research Laboratory and the U. S. Army Research Office. Shuting Han was a Howard Hughes Medical Institute International Student Research Fellow.
Source:
Data Science Institute at Columbia
Media Contacts:
Robert Florida – Data Science Institute at Columbia
Image Source:
The image is credited to Rafael Yuste et al.
Original Research: Open access
“Reduced Repertoire of Cortical Microstates and Neuronal Ensembles in Medically Induced Loss of Consciousness”. Rafael Yuste et al. Cell Systems. doi:10.1016/j.cels.2019.03.007
Abstract
Reduced Repertoire of Cortical Microstates and Neuronal Ensembles in Medically Induced Loss of Consciousness
Highlights
• Cellular resolution population recordings of mLOC in mice and humans
• mLOC is associated with a decreased cortical repertoire of discriminable microstates
• Neuronal ensemble fragmentation toward independent single neuron activity during mLOC
Summary
Medically induced loss of consciousness (mLOC) during anesthesia is associated with a macroscale breakdown of brain connectivity, yet the neural microcircuit correlates of mLOC remain unknown. To explore this, we applied different analytical approaches (t-SNE/watershed segmentation, affinity propagation clustering, PCA, and LZW complexity) to two-photon calcium imaging of neocortical and hippocampal microcircuit activity and local field potential (LFP) measurements across different anesthetic depths in mice, and to micro-electrode array recordings in human subjects. We find that in both cases, mLOC disrupts population activity patterns by generating (1) fewer discriminable network microstates and (2) fewer neuronal ensembles. Our results indicate that local neuronal ensemble dynamics could causally contribute to the emergence of conscious states.






