A small peptide generated from a collagen protein may protect the brain from schizophrenia by promoting the formation of neuronal synapses, according to a paper published in The Journal of Cell Biology. The study, “Collagen-Derived Matricryptins Promote Inhibitory Nerve Terminal Formation in the Developing Neocortex” by Jianmin Su and colleagues, may lead to new approaches to treating the mental disorder.
The collagen family of extracellular matrix proteins performs numerous functions in the brain, and mutations in several family members cause neurological diseases in humans. How collagen XIX promotes normal brain function is unknown, but loss of the gene encoding this collagen has been linked to familial schizophrenia.
A team of researchers led by Michael Fox at Virginia Tech Carilion Research Institute has been closely examining collagen XIX. They found that collagen XIX-deficient mice display a number of schizophrenia-related symptoms, including an abnormal startle response and an increased susceptibility to seizures. Schizophrenia has previously been linked to defects in a particular type of interneuron. This interneuron dampens neuronal activity in the brain’s cortex by forming inhibitory synapses with the cell bodies of other neurons. These inhibitory synapses were lost in collagen XIX-deficient mice.
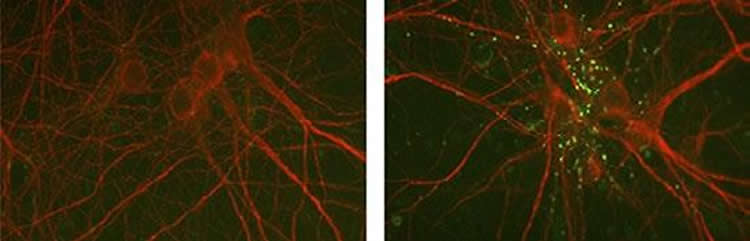
Like similar types of collagen, collagen XIX can be cleaved by extracellular protease enzyme to generate a small signaling peptide called a matricryptin. Jianmin Su and colleagues found that this peptide was sufficient to rescue the formation of inhibitory synapses in neuronal cultures prepared from collagen XIX-deficient mice, apparently by binding and activating a cell adhesion receptor called integrin α5β1.
Fox and colleagues now want to learn more about how collagen XIX’s matricryptin fragment promotes synapse formation. “We also want to investigate whether the peptide holds any therapeutic potential for any diseases that result for malformed or malfunctioning cortical interneurons,” Fox says.
Funding: This study was funded by Brain and Behavior Research Foundation, National Institutes of Health, Virginia Tech Carilion Research Institute.
Source: Ben Short – Rockefeller University Press
Image Source: The image is credited to Su et al.
Original Research: Abstract for “Collagen-derived matricryptins promote inhibitory nerve terminal formation in the developing neocortex” by Jianmin Su, Jiang Chen, Kumiko Lippold, Aboozar Monavarfeshani, Gabriela Lizana Carrillo, Rachel Jenkins, and Michael A. Fox in Journal of Cell Biology. Published online March 2016 doi:10.1083/jcb.201509085
Abstract
Collagen-derived matricryptins promote inhibitory nerve terminal formation in the developing neocortex
Inhibitory synapses comprise only ∼20% of the total synapses in the mammalian brain but play essential roles in controlling neuronal activity. In fact, perturbing inhibitory synapses is associated with complex brain disorders, such as schizophrenia and epilepsy. Although many types of inhibitory synapses exist, these disorders have been strongly linked to defects in inhibitory synapses formed by Parvalbumin-expressing interneurons. Here, we discovered a novel role for an unconventional collagen—collagen XIX—in the formation of Parvalbumin+ inhibitory synapses. Loss of this collagen results not only in decreased inhibitory synapse number, but also in the acquisition of schizophrenia-related behaviors. Mechanistically, these studies reveal that a proteolytically released fragment of this collagen, termed a matricryptin, promotes the assembly of inhibitory nerve terminals through integrin receptors. Collectively, these studies not only identify roles for collagen-derived matricryptins in cortical circuit formation, but they also reveal a novel paracrine mechanism that regulates the assembly of these synapses.
“Collagen-derived matricryptins promote inhibitory nerve terminal formation in the developing neocortex” by Jianmin Su, Jiang Chen, Kumiko Lippold, Aboozar Monavarfeshani, Gabriela Lizana Carrillo, Rachel Jenkins, and Michael A. Fox in Journal of Cell Biology. Published online March 2016 doi:10.1083/jcb.201509085






