Summary: Acute inflammation of the anterior cerebellum resulted in depression-like symptoms in rodent models. Suppressing microglia helped reduce hyperexcitability and symptoms of depression.
Source: Kyoto University
Consider the cerebellum. That structure tucked into the lower back of your skull. Also known as the ‘little brain’ it plays a key role in regulating voluntary movement like balance, motor learning, and speech..
Recent evidence even shows the cerebellum involved in higher-order brain functions including visual response, emotion, and motor planning. And now, a team from Kyoto University has found another link, depressive behavior.
Writing in Cell Reports, the research team found — through a series of experiments with rats — that acute cerebellar inflammation puts the structure in an ‘overexcited’ state, resulting in the animal developing a temporary decrease in motivation and sociability.
Team leader Gen Ohtsuki of Kyoto University’s Hakubi Center for Advanced Research explains that the investigation began in an effort to understand how the brain’s immune system can change its activity. In fact, literature has shown correlations between cerebellar dysfunction and certain pervasive developmental disorders such as autism and depression.
“Even though we now know more about the cerebellum’s role in higher order brain functions the detailed signal transduction machinery remains a mystery. We know even less about what happens in the brain during excessive immune activity.” explains Ohtsuki. “So, we conducted series of experiments where we activated the immune cells in the cerebellum and observed the results.”
The brain’s immune cells are known as microglia, and they respond to bacteria and viruses to mitigate damage. That response results in inflammation. Utilizing electrophysiological techniques, the team found that microglia caused neurons to fire at an increased rate, a phenomenon known as ‘intrinsic-plasticity’. This in turn caused the cerebellum to go into a ‘hyperexcited’ state.
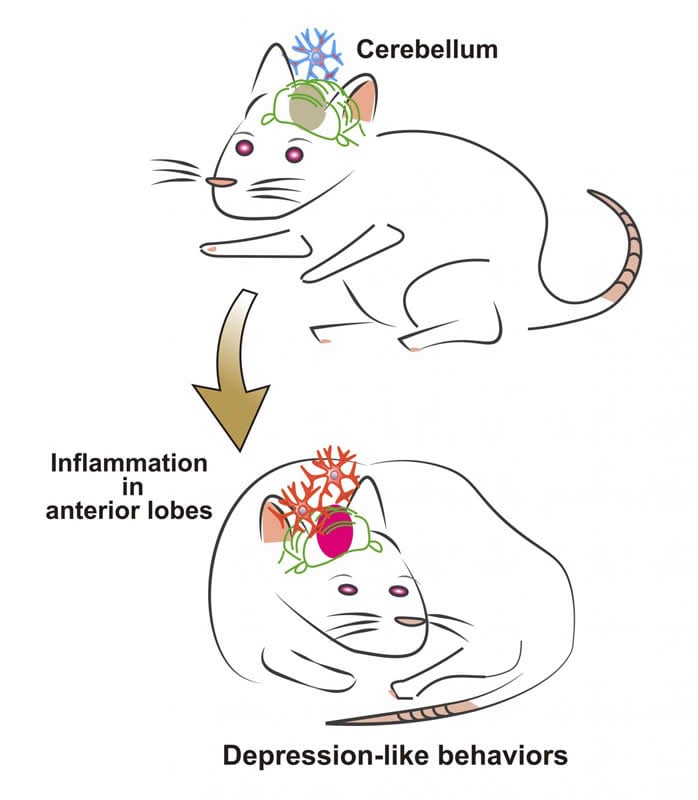
This immune-triggered response was shown to even change behavior. When rats were induced with acute cerebellar inflammation, their sociability, free-searching, and motivation dramatically decreased.
“These behavioral modulations are signs of ‘depression-like’ behavior. Once the inflammation subsided, they were back to normal,” Ohtsuki continues. “Moreover, the phenotype can be rescued if the rats are treated with neuro-immunity suppressants and inflammatory cytokines. We also investigated if higher order brain regions were affected. fMRI studies on the rats show a clear increase in activity in the prefrontal cortex, highlighting the interconnectedness of the cerebellum to higher order brain regions.”
The team is encouraged of their results, but states that further investigation is needed.
“Excessive immune activity in the brain can induce behavioral pathology, and we expect it to be involved in other mental and cognitive disorders such as dementia. But to understand anything about the pathological mechanisms we need to combine this with additional data such as genetic risk factors,” concludes Ohtsuki. “In this study, we focused on inflammation. In the future, we will begin firmly clarifying the physiological, molecular, and genetic aspects of these behavioral changes.”
Source:
Kyoto University
Media Contacts:
Raymond Kunikane Terhune – Kyoto University
Image Source:
The image is credited to Kyoto University/Gen Ohtsuki.
Original Research: Closed access
“Microglia-Triggered Plasticity of Intrinsic Excitability Modulates Psychomotor Behaviors in Acute Cerebellar Inflammation”. Gen Ohtsuki et al.
Cell Reports doi:10.1016/j.celrep.2019.07.078
Abstract
Microglia-Triggered Plasticity of Intrinsic Excitability Modulates Psychomotor Behaviors in Acute Cerebellar Inflammation
Highlights
• Purkinje cell-intrinsic plasticity is induced by TNF-α released from microglia
• TNF-α and ATP following microglial activation facilitate synaptic transmission
• Acute inflammation in the anterior cerebellum resulted in depression-like behaviors
• Suppression of microglia rescued both hyperexcitability and behavioral modulation
Summary
Cerebellar dysfunction relates to various psychiatric disorders, including autism spectrum and depressive disorders. However, the physiological aspect is less advanced. Here, we investigate the immune-triggered hyperexcitability in the cerebellum on a wider scope. Activated microglia via exposure to bacterial endotoxin lipopolysaccharide or heat-killed Gram-negative bacteria induce a potentiation of the intrinsic excitability in Purkinje neurons, which is suppressed by microglia-activity inhibitor and microglia depletion. An inflammatory cytokine, tumor necrosis factor alpha (TNF-α), released from microglia via toll-like receptor 4, triggers this plasticity. Our two-photon FRET ATP imaging shows an increase in ATP concentration following endotoxin exposure. Both TNF-α and ATP secretion facilitate synaptic transmission. Region-specific inflammation in the cerebellum in vivo shows depression- and autistic-like behaviors. Furthermore, both TNF-α inhibition and microglia depletion revert such behavioral abnormality. Resting-state functional MRI reveals overconnectivity between the inflamed cerebellum and the prefrontal neocortical regions. Thus, immune activity in the cerebellum induces neuronal hyperexcitability and disruption of psychomotor behaviors in animals.






