Summary: Researchers have created an artificial enzyme that stops alpha-synuclein from spreading. The findings could have positive implications for the treatment of Parkinson’s disease.
Source: Johns Hopkins Medicine
A growing body of research has shown that misshapen and misfolded alpha-synuclein, the protein culprit behind Parkinson’s disease and its characteristics, travels from the gut to the brain, where it spreads and sticks together in lethal clumps known as Lewy bodies. As these clumps accumulate, they cause brain cell death.
Now, Johns Hopkins Medicine researchers have created an artificial enzyme that stops misfolded alpha-synuclein from spreading and could become the basis for a new treatment for Parkinson’s disease.
The results were announced in a study published online Nov. 20, 2020, in the journal Nano Today.
The artificial enzymes, nanosized (a nanometer is a billionth of a meter) combinations of platinum and copper called PtCu bimetallic nanoalloys, were created by the research team for their strong antioxidant properties. The antioxidant capability is dependent largely on the alloy composition.
“Oxidative stress caused by reactive oxygen species is inescapable, and increases with age due to mechanistic slowdowns in processes such as protein degradation,” says senior study researcher Xiaobo Mao, Ph.D., assistant professor of neurology at the Johns Hopkins University School of Medicine. “This indicates the importance of antioxidants, because in Parkinson’s disease, roaming reactive oxygen species promote the spread of misfolded alpha-synuclein, leading to worse symptoms.”
When injected into the brain, the nanozymes scavenge for reactive oxygen species, gobbling them up and preventing them from causing damage to neurons in the brain. The nanozymes mimic catalase and superoxide dismutase, two enzymes found in our bodies that break down reactive oxygen species. Adding the nanozymes strengthens our body’s response to them.
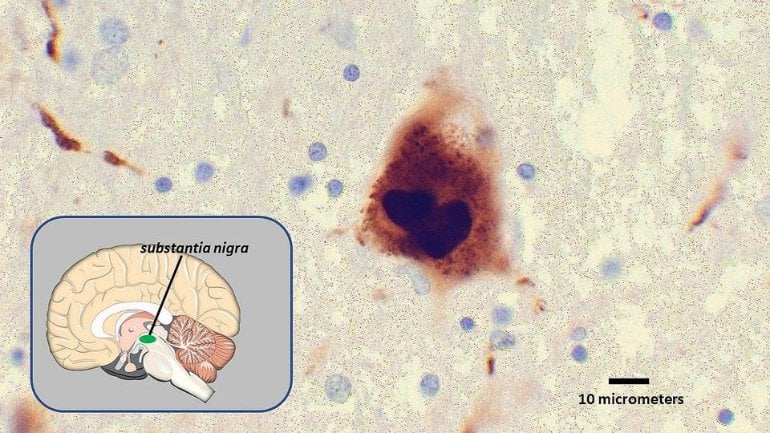
The study used a research method known as the alpha-synuclein preformed fibril model, which replicates the pathology, spreading and neurodegeneration resulting from Lewy bodies. The nanozyme was found to decrease alpha-synuclein induced pathology and inhibit neurotoxicity, in addition to decreasing reactive oxygen species. The nanozyme also prevented alpha-synuclein from passing from cell to cell, and from the substantia nigra to the dorsal striatum, two areas in the midbrain that influence movement and cognition.
Mao has long collaborated with fellow Parkinson’s disease expert Ted Dawson, M.D., Ph.D., professor of neurology and director of the Institute for Cell Engineering at the Johns Hopkins University School of Medicine. Dawson recently added to evidence that misfolded alpha-synuclein travels along the vagus nerve from the gut to the brain. Mao hopes that further research can connect the two findings and lead to a Parkinson’s disease treatment that targets the gut.
“We know that the nanoenzymes work when injected directly into the brain,” says Mao. “Now, we’d like to see if the nanoenzymes can block the disease progression induced by pathogenic alpha-synuclein traveling from the gut, across the blood-brain barrier and into the brain.”
About this Parkinson’s disease research news
Source: Johns Hopkins Medicine
Contact: Ayanna Tucker – Johns Hopkins Medicine
Image: The image is credited to M.E. Newman, Johns Hopkins Medicine
Original Research: Closed access.
“Nanozyme scavenging ROS for prevention of pathologic α-synuclein transmission in Parkinson’s disease” by Xiaobo Mao et al. Nano Today
Abstract
Nanozyme scavenging ROS for prevention of pathologic α-synuclein transmission in Parkinson’s disease
Braak’s prion-like theory fundamentally subverts the understanding of Parkinson’s disease (PD). Emerging evidence shows that pathologic α-synuclein (α-syn) is a prion-like protein that spreads from one region to another in PD brain, which is an essential driver to the pathogenesis of PD. Thus far, there is a big knowledge gap that limited nanomaterial that can block prion-like spreading. Here, α-syn preformed fibrils (PFF) are used to model prion-like spreading and biocompatible antioxidant nanozyme, PtCu nanoalloys (NAs), is applied to fight against α-syn spreading. The results show that PtCu NAs significantly inhibit α-syn pathology, cell death, and neuron-to-neuron transmission by scavenging reactive oxygen species (ROS) in primary neuron cultures. Moreover, the PtCu NAs significantly inhibit α-syn spreading induced by intrastriatal injection of PFF. It is the first time to observe nanozyme can block prion-like spreading, which provides a proof of concept for nanozyme therapy. It is also anticipated that the biomedical application of nanozyme against prion-like spreading could be optimized and considered to be developed as a therapeutic strategy against Parkinson’s disease, Alzheimer’s disease, and other prion-like proteinopathies.







