A team led by scientists from The Scripps Research Institute (TSRI) has found a simple method to convert human skin cells into the specialized neurons that detect pain, itch, touch and other bodily sensations. These neurons are also affected by spinal cord injury and involved in Friedreich’s ataxia, a devastating and currently incurable neurodegenerative disease that largely strikes children.
The discovery allows this broad class of human neurons and their sensory mechanisms to be studied relatively easily in the laboratory. The “induced sensory neurons” generated by this method should also be useful in the testing of potential new therapies for pain, itch and related conditions.
“Following on the work of TSRI Professor Ardem Patapoutian, who has identified many of the genes that endow these neurons with selective responses to temperature, pain and pressure, we have found a way to produce induced sensory neurons from humans where these genes can be expressed in their ‘normal’ cellular environment,” said Associate Professor Kristin K. Baldwin, an investigator in TSRI’s Dorris Neuroscience Center. “This method is rapid, robust and scalable. Therefore we hope that these induced sensory neurons will allow our group and others to identify new compounds that block pain and itch and to better understand and treat neurodegenerative disease and spinal cord injury.”
The report by Baldwin’s team appears as an advance online publication in Nature Neuroscience on November 24, 2014.
In Search of a Better Model
The neurons that can be made with the new technique normally reside in clusters called dorsal root ganglia (DRG) along the outer spine. DRG sensory neurons extend their nerve fibers into the skin, muscle and joints all over the body, where they variously detect gentle touch, painful touch, heat, cold, wounds and inflammation, itch-inducing substances, chemical irritants, vibrations, the fullness of the bladder and colon, and even information about how the body and its limbs are positioned. Recently these neurons have also been linked to aging and to autoimmune disease.
Because of the difficulties involved in harvesting and culturing adult human neurons, most research on DRG neurons has been done in mice. But mice are of limited use in understanding the human version of this broad “somatosensory” system.
“Mouse models don’t represent the full diversity of the human response,” said Joel W. Blanchard, a PhD candidate in the Baldwin laboratory who was co-lead author of the study with Research Associate Kevin T. Eade.
A New Identity
For the new study, the team used a cell-reprogramming technique (similar to those used to reprogram skin cells into stem cells) to generate human DRG-type sensory neurons from ordinary skin cells called fibroblasts.
To start, the scientists examined previous experiments and identified several transcription factors—managerial proteins that switch on the activity of large sets of genes—that seemed crucial to the ability of immature neurons to develop into adult sensory neurons. They found that the combination of the transcription factors Brn3a plus Ngn1, or Brn3a plus Ngn2, reprogrammed a significant percentage of the embryonic mouse fibroblasts into what looked—and acted—like mature DRG-type sensory neurons.
“We added compounds including capsaicin, which activates pain receptors on DRG neurons, and menthol, which activates cold receptors, and saw subsets of our induced neurons light up with activity just as real DRG neurons would,” said Eade.
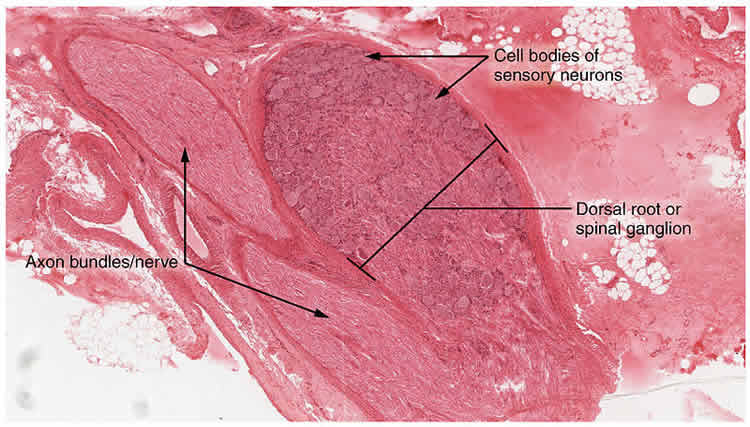
Remarkably, although mouse studies had indicated that different transcription factors were differently important for generating pain and itch sensing neurons versus pressure and limb position neurons, in the dish these factors produced equal numbers of each of the three main subtypes.
A Step Toward ‘Personalized Medicine’
Using the same recipes of transcription factors, the team was able to convert adult human fibroblasts, which are harder to reprogram, into DRG neurons. The conversion rate was lower, but the induced neurons seemed just as much like their natural counterparts as those produced from embryonic mouse fibroblasts.
“We can definitely scale up of the numbers of these induced neurons as needed,” Blanchard said.
The feat means that scientists now can relatively easily study DRG sensory neurons derived from many different people, to better understand the diversity of human sensory responses and sensory disorders and advance a “personalized medicine” approach. “We can start to understand how individuals respond uniquely to pain, cold, itch and so on,” said Blanchard.
Other co-authors of the study, “Selective conversion of fibroblasts into peripheral sensory neurons,” were Valentina Lo Sardo, Rachel K. Tsunemoto, Daniel Williams, and Pietro Paolo Sanna, all of TSRI; and Attila Szűcs of the University of California San Diego, who performed many of the electrical tests on the induced neurons.
Support for the research came from the Dorris Neuroscience Center, the California Institute of Regenerative Medicine, the Baxter Family Foundation, the Del Webb Foundation, The Norris Foundation, Las Patronas, the National Institutes of Health (National Institute on Drug Abuse [DA031566], National Institute on Deafness and other Communication Disorders [DC012592] and National Institute of Mental Health [MH102698]), the National Science Foundation and the Andrea Elizabeth Vogt Memorial Award.
Contact: Office of Communications – Scripps Research Institute
Source: Scripps Research Institute press release
Image Source: The image is credited to OpenStax College and is licensed Creative Commons Attribution 3.0 Unported
Original Research: Abstract for “Selective conversion of fibroblasts into peripheral sensory neurons” by Joel W Blanchard, Kevin T Eade, Attila Szűcs, Valentina Lo Sardo, Rachel K Tsunemoto, Daniel Williams, Pietro Paolo Sanna and Kristin K Baldwin in Nature Neuroscience. Published online November 24 2014 doi:10.1038/nn.3887






