Sometimes, a nematode worm just needs to take a nap. In fact, its life may depend on it. New research has identified a protein that promotes a sleep-like state in the nematode Caenorhabditis elegans. Without the snooze-inducing molecule, worms are more likely to die when confronted with stressful conditions, report researchers in the March 7, 2016 issue of the journal GENETICS.
The C. elegans sleep-like behavior is surprisingly similar to the sleep of humans and other mammals. In this state, the worm stops moving, relaxes, and uncurls its body. It also shows reduced neuronal activity and is less responsive to stimuli, but will then”wake up” if an experimenter pokes it too much. Like sleep-deprived humans, a worm that has been woken up repeatedly will fall back to sleep faster and will stay asleep longer than a well-rested worm.
The similarities are not just superficial: many of the molecules that regulate this process in C. elegans, including the epidermal growth factor receptor (EGFR), also influence sleep in mammals. This suggests that the worm sleep-like state is evolutionarily related to our own slumber. Because C. elegans is easy to experimentally manipulate and has a simple nervous system, it can serve as a tool for understanding how human sleep-wake cycles are regulated.
Worms don’t sleep on a day/night schedule like mammals. Instead, their sleep-like behavior occurs at specific stages during development; the worms enter this state each time they transition from one larval stage to another. They also sleep for several hours after a stressful event, including extremely hot or cold conditions or exposure to toxins. If they don’t get this post-stress nap, worms are less likely to survive the noxious event.
In the new research, the authors investigated whether a protein known to regulate rhythmic activities in C. elegans–like feeding, defecating, and reproducing–also regulates sleep. This protein, VAV-1, is active in a specific worm neuron that has previously been implicated in promoting sleep-like states via EGFR.
The researchers found that VAV-1 was needed specifically in the sleep-regulating neuron for sleep after stress, but not for the type of sleep linked to larval transitions. They also found that VAV-1 is needed for maintaining normal amounts of EGFR in the neuron. The sleep state could be directly induced by genetically modifying the worms so that they overproduce VAV-1.
Crucially, VAV-1 is needed for the protective effects of post-stress sleep: after being heated to a sweltering 40°C (104°F), worms lacking VAV-1 function were substantially more likely to die within the following days.
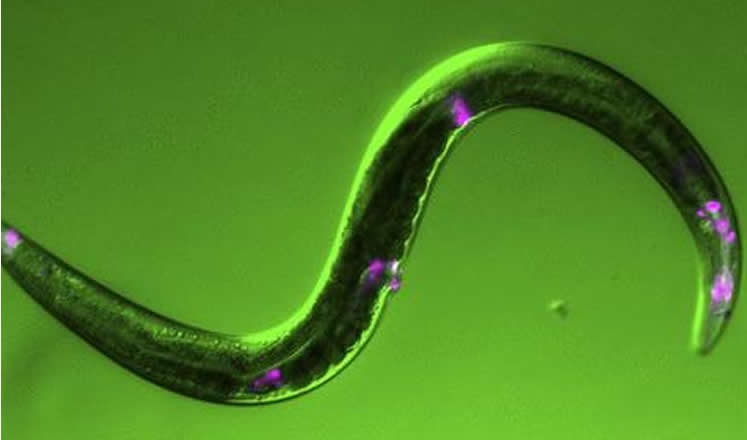
It remains unknown whether the human equivalent of VAV-1 plays a role in regulating human sleep, but the idea is worth further investigation, says study leader Kenneth Norman, of Albany Medical College. The human proteins Vav2 and Vav3 are expressed in the thalamus, a brain structure involved in regulating sleep, and, like worm VAV-1, the mammalian proteins seem to play a role in nervous system function and EGFR signaling.
“Just as in mammals, the nematode sleep program is incredibly important for life. The mystery remains what is happening during sleep that allows animals to survive,” says Norman. “We hope we can find some valuable clues by studying this evolutionarily-conserved process in a powerful model organism like C. elegans.”
Funding: This work was supported by NIH grants GM088213 and NS055813, a BIBS/NPNI Postdoctoral Fellowship in Translational Neuroscience, and resources provided by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Source: Cristy Gelling – Genetics Society of America
Image Credit: The image is credited to Amanda Fry and Ken Norman.
Original Research: Full open access research for “A Conserved GEF for Rho-Family GTPases Acts in an EGF Signaling Pathway to Promote Sleep-like Quiescence in Caenorhabditis elegans” by Amanda L. Fry, Jocelyn T. Laboy, Huiyan Huang, Anne C. Hart, and Kenneth R. Norman in Genetics. Published online March 7 2016 doi:10.1534/genetics.115.183038
Abstract
A Conserved GEF for Rho-Family GTPases Acts in an EGF Signaling Pathway to Promote Sleep-like Quiescence in Caenorhabditis elegans
Sleep is evolutionarily conserved and required for organism homeostasis and survival. Despite this importance, the molecular and cellular mechanisms underlying sleep are not well understood. Caenorhabditis elegans exhibits sleep-like behavioral quiescence and thus provides a valuable, simple model system for the study of cellular and molecular regulators of this process. In C. elegans, epidermal growth factor receptor (EGFR) signaling is required in the neurosecretory neuron ALA to promote sleep-like behavioral quiescence after cellular stress. We describe a novel role for VAV-1, a conserved guanine nucleotide exchange factor (GEF) for Rho-family GTPases, in regulation of sleep-like behavioral quiescence. VAV-1, in a GEF-dependent manner, acts in ALA to suppress locomotion and feeding during sleep-like behavioral quiescence in response to cellular stress. Additionally, VAV-1 activity is required for EGF-induced sleep-like quiescence and normal levels of EGFR and secretory dense core vesicles in ALA. Importantly, the role of VAV-1 in promoting cellular stress–induced behavioral quiescence is vital for organism health because VAV-1 is required for normal survival after cellular stress.
“A Conserved GEF for Rho-Family GTPases Acts in an EGF Signaling Pathway to Promote Sleep-like Quiescence in Caenorhabditis elegans” by Amanda L. Fry, Jocelyn T. Laboy, Huiyan Huang, Anne C. Hart, and Kenneth R. Norman in Genetics. Published online March 7 2016 doi:10.1534/genetics.115.183038