Summary: Researchers have identified a direct cellular interaction between the nervous system and the immune system. Pain sensing neurons around the lymph nodes can modulate lymph node activity.
Source: Broad Institute
The nervous and immune systems have long been thought to be separate entities in the body, but new research has uncovered a direct cellular interaction between the two. Scientists from Harvard Medical School, the Broad Institute of MIT and Harvard, MIT, and the Ragon Institute of MGH, MIT and Harvard have found that pain-sensing neurons surround lymph nodes in mice, and can modulate the activity of these small organs, which are key parts of the immune system.
The new research, published in Cell, reveals the cells that mediate the crosstalk between the nervous and immune systems. It also paves the way for more research on how the nervous system regulates immune responses, for example, during an infection.
“Once you begin to understand how these systems are structured and what are the mechanisms by which they perform their biological handshakes, it really opens up some tremendous opportunities to think about how to rationally modulate those interactions as a possible therapeutic strategy,” said Alex Shalek, co-senior author of the study, a Broad institute member, a Ragon member, and an associate professor of chemistry, a core member of the Institute for Medical Engineering and Science, and an extramural member of the Koch Institute for Integrative Cancer Research at MIT.
Ulrich von Andrian, a professor of immunopathology at Harvard Medical School and a program leader at the Ragon Institute, is co-senior author of the study. Siyi Huang, a postdoctoral fellow in von Andrian’s lab, is a co-first author of the paper.
ILLUMINATING THE LYMPH NODE
Previous research had pointed to a possible connection between neurons and lymph nodes. In the new study, the research team used a variety of methods, including advanced imaging, retrograde tracing, single-cell RNA sequencing, and optogenetics, to map the connections between neurons, immune and other types of cells within lymph nodes in mice. They found that sensory neurons that originate in a region of the spinal cord called the dorsal root ganglion formed a mesh that innervated, or made connections, throughout the periphery of the lymph node.
Out of the thousands of sensory cells in the dorsal root ganglion, the group identified only a handful that contribute to this coupling in a single lymph node. “We found the 20 or so needles in a haystack that are innervating a lymph node,” said Carly Ziegler, a graduate student with Harvard University and MIT who is in Shalek’s lab and is a co-first author of the study. “Identifying and picking out that specific cell body in a diverse set of neurons was really challenging.”
By using single-cell RNA sequencing to analyze the gene expression of these sensory cells, the team discovered that the cells were mainly pain-sensing neurons. Further analysis revealed that these neurons were highly diverse in the genes they expressed, and that they expressed different synaptic proteins and cell surface molecules than sensory neurons that innervate the skin, suggesting different modes of cell-to-cell communication.
To confirm whether the neurons were interacting with the lymph node, the researchers induced artificial immune responses in the mice and found that the pain-sensing neurons responded by increasing their density within the enlarging lymph node. This suggests that the sensory neurons can sense and effectively respond to changes in the lymph node.
The scientists also found that this communication was two-way: the neurons themselves can modulate cells in the lymph node. The researchers used optogenetics to activate these neurons and observed gene expression changes in specific cells of the lymph node, including endothelial cells.
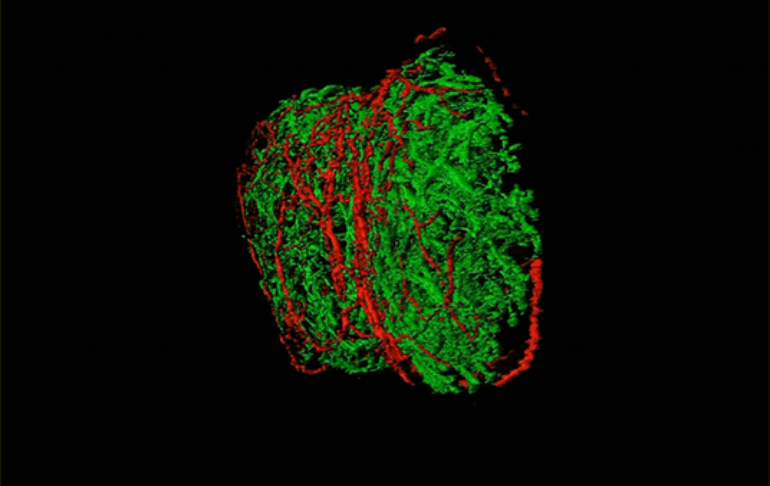
The finding indicates that these neurons are not mere spectators. “We found that they actually can change a lot of the local environment of the lymph node and seem to be specifically influencing particular cells and structures,” said Ziegler.
The researchers are continuing to learn more about the role of these neurons in the lymph nodes, such as which mechanisms they are using to interact with the lymph nodes, whether they are mobilizing specific parts of the immune system, and what happens if these cellular circuits are lacking or dysfunctional.
The study also provides a framework for researchers to dissect the connections between other systems in the body. “We’re starting to figure out how to put together toolkits that come from multiple fields,” Shalek said. “That will help us gain new biological insights into how our bodies work as one single integrated system.”
Funding: Support for this research was provided in part by the National Institutes of Health, the Searle Scholars Program, the Beckman Young Investigator Program, the Pew-Stewart Scholars Program for Cancer Research, a Sloan Fellowship in Chemistry, the Ragon Institute of MGH, MIT and Harvard, and the HHMI Damon Runyon Cancer Research Foundation Fellowship.
About this neuroscience research news
Source: Broad Institute
Contact: Press Office – Broad Institute
Image: The image is credited to Siyi Huang, John Austin, Najat Mannoun
Original Research: Closed access.
“Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential” by Alex Shalek et al. Cell
Abstract
Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential
Highlights
- •Anatomical and molecular identification of lymph node-innervating nociceptors
- •Demonstration of inflammation-induced plasticity of sensory innervation of lymph nodes
- •Transcriptome-based identification of nociceptor target cells in lymph node
- •Optogenetic validation of lymph node target cell modulation by nociceptor activity
Summary
Barrier tissue immune responses are regulated in part by nociceptors. Nociceptor ablation alters local immune responses at peripheral sites and within draining lymph nodes (LNs). The mechanisms and significance of nociceptor-dependent modulation of LN function are unknown. Using high-resolution imaging, viral tracing, single-cell transcriptomics, and optogenetics, we identified and functionally tested a sensory neuro-immune circuit that is responsive to lymph-borne inflammatory signals. Transcriptomics profiling revealed that multiple sensory neuron subsets, predominantly peptidergic nociceptors, innervate LNs, distinct from those innervating surrounding skin. To uncover LN-resident cells that may interact with LN-innervating sensory neurons, we generated a LN single-cell transcriptomics atlas and nominated nociceptor target populations and interaction modalities. Optogenetic stimulation of LN-innervating sensory fibers triggered rapid transcriptional changes in the predicted interacting cell types, particularly endothelium, stromal cells, and innate leukocytes. Thus, a unique population of sensory neurons monitors peripheral LNs and may locally regulate gene expression.






