Summary: In males, a genetic variant of DUSP8 can increase the risk of type 2 diabetes by impairing the brain’s response to insulin.
Source: DZD
The brain plays a major role in controlling our blood glucose levels. In type 2 diabetics this glucose metabolism brain control is often dysfunctional. Genetic components for this phenomenon have so far remained elusive. A group of scientists at Helmholtz Zentrum München and the German Center for Diabetes Research (DZD) have now shown in the Journal of Clinical Investigations that in men a genetic variant of the gene DUSP8 can increase the risk for type 2 diabetes by impairing our brain response to the hormone insulin.
Insulin is a pancreatic hormone that controls our blood sugar levels. Insulin not only stimulates the uptake of glucose from blood into peripheral tissues, it also acts on the brain, specifically the hypothalamus, to control glucose and energy metabolism. In obese subjects, insulin loses some of its activity due to the activation of inflammatory signaling and a subsequent impairment of the cellular response downstream to the insulin receptor. This phenomenon, termed insulin resistance, is a major hallmark in the development of type 2 diabetes, but to date not yet fully understood. Recent genome-wide association studies (GWAS) identified DUSP8 as type 2 diabetes risk gene. Now, scientists have investigated how the protein Dusp8 (dual-specificity phosphatase 8), which is encoded by the DUSP8 gene, regulates glucose tolerance and insulin sensitivity.
Genetic variant of the DUSP8 gene increases risk of type 2 diabetes
“Carriers of a genetic variant of the gene DUSP8 were shown to have a moderately increased risk for type 2 diabetes, but the functional importance of Dusp8 for the etiology of the disease remained unknown,” explains Dr. Sonja C. Schriever, lead author of the study. “By combining cellular models, Dusp8 loss- and gain-of-function mice and functional magnetic resonance imaging (fMRI) of humans with genetic variants in the gene DUSP8, we now exposed a specific role of the protein Dusp8 as a gatekeeper for systemic glucose tolerance and hypothalamic insulin sensitivity,” adds colleague and senior author Dr. Paul Pfluger. Within the framework of the German Center for Diabetes Research (DZD), both scientists formed a team of biologists, epidemiologists and clinicians that was able to unravel the molecular mechanisms that link Dusp8 with the development of type 2 diabetes.
Protein Dusp8 has regulatory effects on hypothalamic insulin sensitivity
The protein Dusp8 has a regulatory effect on inflammatory processes in the hypothalamus and on the hypothalamic-pituitary-adrenal (HPA) axis. It seems to protect the organism against hyper-activation of inflammatory signaling and impaired insulin sensitivity in the hypothalamus. The scientists could show that the deletion of the Dusp8 gene in male but not in female mice increased hypothalamic inflammation, impaired HPA axis feedback and increased basal stress hormone levels, which all together aggravated the insulin sensitivity. The sex-specific role of murine Dusp8 was consistent with fMRI data in human volunteers that revealed reduced hypothalamic insulin sensitivity in male but not female carriers of the DUSP8 type 2 diabetes risk variant.
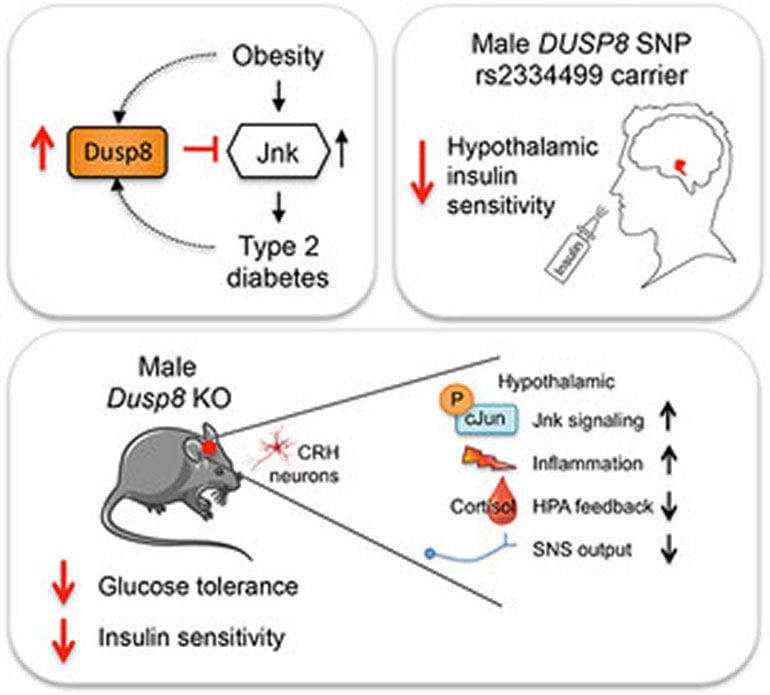
“Unraveling the multi-systemic processes that drive the impaired hypothalamic insulin sensitivity in the mouse models was an important step to understand the mechanistic underpinnings of the type 2 diabetes risk gene DUSP8”, concludes Schriever. In future studies, the researchers want to investigate the effect of central insulin action and the DUSP8 type 2 diabetes risk variant on the hypothalamic-pituitary-adrenal axis in human subjects with or without T2D.
About this neurology research article
Source:
DZD
Contacts:
Paul Pfluger – DZD
Image Source:
The image is credited to Helmholtz Zentrum München.
Original Research: Closed access
“Type 2 diabetes risk gene Dusp8 regulates hypothalamic Jnk signaling and insulin sensitivity” by Schriever SC et. al. Journal of Clinical Investigation.
Abstract
Type 2 diabetes risk gene Dusp8 regulates hypothalamic Jnk signaling and insulin sensitivity
Recent genome-wide association studies (GWAS) identified DUSP8, a dual-specificity phosphatase targeting MAP kinases, as type 2 diabetes (T2D) risk gene. Here, we unravel Dusp8 as gatekeeper in the hypothalamic control of glucose homeostasis in mice and humans. Male but not female Dusp8 loss-of-function mice, either with global or CRH neuron-specific deletion, had impaired systemic glucose tolerance and insulin sensitivity when exposed to high-fat diet (HFD). Mechanistically, we found impaired hypothalamic–pituitary–adrenal (HPA) axis feedback, blunted sympathetic responsiveness, and chronically elevated corticosterone levels driven by hypothalamic hyperactivation of Jnk signaling. Accordingly, global Jnk1 ablation, AAV-mediated Dusp8 overexpression in the mediobasal hypothalamus, or metyrapone-induced chemical adrenalectomy rescued the impaired glucose homeostasis of obese male Dusp8 KO mice, respectively. The sex-specific role of murine Dusp8 in governing hypothalamic Jnk signaling, insulin sensitivity and systemic glucose tolerance was consistent with fMRI data in human volunteers that revealed an association of the DUSP8 rs2334499 risk variant with hypothalamic insulin resistance in men. Further, expression of DUSP8 was increased in the infundibular nucleus of T2D humans. In summary, our findings suggest the GWAS-identified gene Dusp8 as novel hypothalamic factor that plays a functional role in the etiology of T2D.






