Summary: Glioblastmoa brain cancer patients who received an experimental vaccines in combination with chemotherapy showed improved survivability and tolerated the treatment well, a new study reports.
Source: Duke University.
A phase one study of 11 patients with glioblastoma who received injections of an investigational vaccine therapy and an approved chemotherapy showed the combination to be well tolerated while also resulting in unexpectedly significant survival increases, researchers at the Duke Cancer Institute report.
A phase one study of 11 patients with glioblastoma who received injections of an investigational vaccine therapy and an approved chemotherapy showed the combination to be well tolerated while also resulting in unexpectedly significant survival increases, researchers at the Duke Cancer Institute report.
Patients treated with the study drug (dose-intensified temozolomide and vaccines) were continuously monitored for toxicity and adverse events. Study patients experienced known side effects with temozolomide, including nausea, lymphopenia, thrombocytopenia and fatigue.
There were no treatment limiting adverse events and no adverse events related to the cellular portion of the vaccine. One patient developed a grade 3 vaccine-related allergic reaction to the GM-CSF component of the vaccine. The patient was able to continue vaccinations in which the GM-CSF was removed and had no subsequent adverse events.
Although the trial was small and not designed to evaluate efficacy, four of the 11 study patients survived for more than five years following treatment with a combination of vaccine and the drug temozolomide, a first-line chemotherapy drug for glioblastoma. That outcome is uncommon for glioblastoma, a lethal brain cancer that has a median survival of nearly 15 months when treated with the current standard of care.
“This is a small study, but it’s one in a sequence of clinical trials we have conducted to explore the use of an immunotherapy that specifically targets a protein on glioblastoma tumors,” said Duke’s Kristen Batich, M.D., Ph.D., lead author of a study published online April 14 in the journal Clinical Cancer Research. “While not a controlled efficacy study, the survival results were surprising, and they suggest the possibility that combining the vaccine with a more intense regimen of this chemotherapy promotes a strong cooperative benefit.”
Batich and colleagues–including senior author John Sampson, M.D., Ph.D., chair of Duke’s Department of Neurosurgery — treated 11 patients as part of a single arm study to test the safety of using a dose-intensified regimen of temozolomide along with a dendritic cell vaccine therapy that selectively targets a cytomegalovirus (CMV) protein. CMV proteins are abundant in glioblastoma tumors, but are absent in surrounding brain cells.
In earlier clinical trials, the researchers used the dendritic cell vaccine to teach T-cells to attack tumor cells, and their data suggested these vaccines could be enhanced when primed by an immune system booster. A separate clinical trial found that higher-than-standard doses of temozolomide, combined with an immune-stimulating factor, also primed the immune system and enhanced the response of a different vaccine target.
The researchers built on those findings in the current study. They used a combination of the dendritic cell vaccine therapy and the immune-stimulating factor, which was administered as injections following dose-intensified regimens of temozolomide. The 11 patients received at least six vaccine treatments.
“Our strategy was to capitalize on the immune deficiency caused by the temozolomide regimen,” Batich said. “It seems counter-intuitive, but when the patient’s lymphocytes are depleted, it’s actually an optimal time to introduce the vaccine therapy. It basically gives the immune system marching orders to mount resources to attack the tumor.”
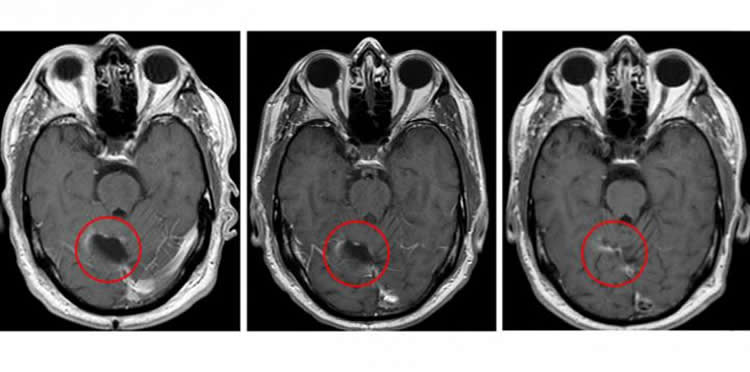
Batich said the approach significantly slowed the progression of patients’ tumors. Typically, glioblastoma tumors begin to regrow after standard treatment at a median of eight months, but for study participants, recurrence occurred at a median of 25 months.
“These are surprisingly promising clinical outcomes,” Sampson said. “However, it is important to emphasize that this was a very small study that used historical comparisons rather than randomizing patients to two different treatments, but the findings certainly support further study of this approach in larger, controlled clinical trials.”
The research team has received approval to launch a new study that will compare the standard dose of temozolomide vs. the dose-intensified regimen along with the vaccine in glioblastoma patients.
In addition to Sampson and Batich, study authors include Elizabeth A. Reap, Gary E. Archer, Luis Sanchez-Perez, Smita K. Nair, Robert J. Schmittling, Pam Norberg, Weihua Xie, James E. Herndon, Patrick Healy, Roger E. McLendon, Allan H. Friedman, Henry S. Friedman, Darell Bigner, Gordana Vlahovic and Duane A. Mitchell.
Funding: The study received funding support from the National Institutes of Health’s Brain Cancer SPORE grant (P50-CA190991-02); Small Business Technology Transfer with Annias Immunotherapeutics, Inc. (R42-CA153845-02); Duke Comprehensive Cancer Core Grant (P30-CA14236-42); and the NIH (R01-CA177476-04, R01-NS085412-04, R01-NS086943-03, R01-NS067037, R01-CA134844, 1R01CA175517-01A1, P01-CA154291-05, CA180411-01, R25-NS065731-07).
Source: Samiha Khanna – Duke University
Image Source: NeuroscienceNews.com image is credited Clinical Cancer Research/American Association for Cancer Research.
Original Research: Full open access research for “Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination” by Kristen A. Batich, Elizabeth A. Reap, Gary E. Archer, Luis Sanchez-Perez, Smita K. Nair, Robert J. Schmittling, Pam Norberg, Weihua Xie, James E. Herndon II, Patrick Healy, Roger E. McLendon, Allan H. Friedman, Henry S. Friedman, Darell Bigner, Gordana Vlahovic, Duane A. Mitchell and John H. Sampson in Clinical Cancer Research. Published online April 13 2017 doi:10.1158/1078-0432.CCR-16-2057
[cbtabs][cbtab title=”MLA”]Duke University “Immunotherapy for Glioblastoma Well Tolerated and Increases Survival.” NeuroscienceNews. NeuroscienceNews, 14 April 2017.
<https://neurosciencenews.com/immunotherapy-glioblastoma-6416/>.[/cbtab][cbtab title=”APA”]Duke University (2017, April 14). Immunotherapy for Glioblastoma Well Tolerated and Increases Survival. NeuroscienceNew. Retrieved April 14, 2017 from https://neurosciencenews.com/immunotherapy-glioblastoma-6416/[/cbtab][cbtab title=”Chicago”]Duke University “Immunotherapy for Glioblastoma Well Tolerated and Increases Survival.” https://neurosciencenews.com/immunotherapy-glioblastoma-6416/ (accessed April 14, 2017).[/cbtab][/cbtabs]
Abstract
Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination
Purpose: Patients with glioblastoma have less than 15-month median survival despite surgical resection, high-dose radiation, and chemotherapy with temozolomide. We previously demonstrated that targeting cytomegalovirus pp65 using dendritic cells (DC) can extend survival and, in a separate study, that dose-intensified temozolomide (DI-TMZ) and adjuvant granulocyte macrophage colony-stimulating factor (GM-CSF) potentiate tumor-specific immune responses in patients with glioblastoma. Here, we evaluated pp65-specific cellular responses following DI-TMZ with pp65-DCs and determined the effects on long-term progression-free survival (PFS) and overall survival (OS).
Experimental Design: Following standard-of-care, 11 patients with newly diagnosed glioblastoma received DI-TMZ (100 mg/m2/d × 21 days per cycle) with at least three vaccines of pp65 lysosome–associated membrane glycoprotein mRNA-pulsed DCs admixed with GM-CSF on day 23 ± 1 of each cycle. Thereafter, monthly DI-TMZ cycles and pp65-DCs were continued if patients had not progressed.
Results: Following DI-TMZ cycle 1 and three doses of pp65-DCs, pp65 cellular responses significantly increased. After DI-TMZ, both the proportion and proliferation of regulatory T cells (Tregs) increased and remained elevated with serial DI-TMZ cycles. Median PFS and OS were 25.3 months [95% confidence interval (CI), 11.0–∞] and 41.1 months (95% CI, 21.6–∞), exceeding survival using recursive partitioning analysis and matched historical controls. Four patients remained progression-free at 59 to 64 months from diagnosis. No known prognostic factors [age, Karnofsky performance status (KPS), IDH-1/2 mutation, and MGMT promoter methylation] predicted more favorable outcomes for the patients in this cohort.
Conclusions: Despite increased Treg proportions following DI-TMZ, patients receiving pp65-DCs showed long-term PFS and OS, confirming prior studies targeting cytomegalovirus in glioblastoma.
Translational Relevance
The highly aggressive and therapeutically resistant nature of glioblastoma is evidenced by a median survival of less than 15 months. More precise and efficacious therapies are desperately needed. Several groups have demonstrated that cytomegalovirus proteins are expressed in more than 90% of sampled glioblastomas. Moreover, cytomegalovirus antigen expression is restricted to glioma cells and not surrounding normal brain, providing the opportunity to subvert cytomegalovirus proteins as tumor-specific immunotherapy targets. In this study, we targeted the cytomegalovirus antigen pp65 using dendritic cells (DC) in combination with dose-intensified temozolomide and evaluated patient antitumor immune responses and survival. Despite increases in regulatory T-cell proportions after temozolomide, patients treated with pp65-DCs showed increased pp65 immunity and long-term survival extending beyond predicted rates, fortifying prior studies that target cytomegalovirus antigens in glioblastoma. Randomized studies on the prevention of generated regulatory T cells in the context of temozolomide treatment and cytomegalovirus targeting are under way.
“Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination” by Kristen A. Batich, Elizabeth A. Reap, Gary E. Archer, Luis Sanchez-Perez, Smita K. Nair, Robert J. Schmittling, Pam Norberg, Weihua Xie, James E. Herndon II, Patrick Healy, Roger E. McLendon, Allan H. Friedman, Henry S. Friedman, Darell Bigner, Gordana Vlahovic, Duane A. Mitchell and John H. Sampson in Clinical Cancer Research. Published online April 13 2017 doi:10.1158/1078-0432.CCR-16-2057