Summary: Researchers use two-photon imaging and optogenetics to isolate and study brain capillaries in animal models.
Source: Seattle Children’s Hospital
While the human brain has over 400 miles of total vasculature, little is known about the tiny capillaries that make up much of this intricate labyrinth of blood vessels critical for delivering oxygenated blood and nutrients to billions of brain cells.
According to Dr. Andy Shih, a principal investigator in the Center for Developmental Biology and Regenerative Medicine at Seattle Children’s Research Institute, understanding how this vast network regulates blood flow in the brain could hold the key to new treatments for neonatal and childhood neurologic conditions, such as stroke and hypoxia, and issues of aging like dementia and Alzheimer’s disease.
“Insufficient blood flow contributes to many of the common neurologic problems seen in children and adults,” he said. “Yet, because we can’t see the capillaries, which measure about 1/10th the thickness of hair, with in vivo clinical imaging techniques, determining how blood travels through this densely packed bed of vessels has remained elusive.”
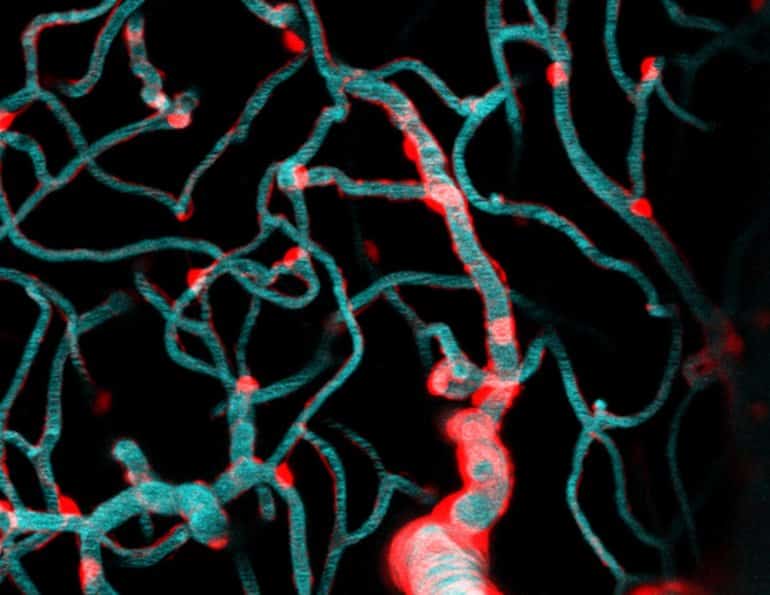
Wanting to get a closer look, Shih and fellow scientists, Dr. Andree-Anne Berthiaume and Dr. David Hartmann, applied special techniques called two-photon imaging and optogenetics to isolate and study brain capillaries in animal models. Their findings published in Nature Neuroscience describe the dynamics that govern capillary blood flow in the brain and have broad implications for future avenues of brain research.
A cell painted on the blood vessel
In the study, the scientific team led by Shih focused on a cell type called a pericyte. Pericytes cover the capillary surface and are broadly categorized as a vascular mural cell.
“They’re called mural cells because they adhere closely to the vessel wall (muralis is Latin for wall), but I to imagine it’s like they’re painted on the capillaries with long brushstrokes” Shih said. “Pericytes live on all of these brain capillaries, contacting more than 90% of this dense network. Unlike mural cells of arteries and arterioles, they have long appendages that don’t surround the whole vessel.”
Scientists have long debated if pericytes are structurally equipped to influence blood flow in the brain capillaries.
“Whether brain capillary pericytes constrict and dilate capillaries has remained a controversial hypothesis since they were discovered,” Shih said. “Research exploring these dynamics in living organisms have resulted in mixed outcomes, with some studies supporting this theory, and others reporting the contrary.”
Shih says there are many reasons brain capillary pericytes are challenging to study in vivo.
“The connectedness of vasculature makes it very difficult to isolate the effects of pericytes from arterioles that strongly, but indirectly, impact capillary blood flow in the brain,” he said. “Capillaries also go undetected by most imaging technology that lack the resolution or are more focused on blood flow in the larger arteries.”
Pinpointing the pericytes
The researchers proposed a specialized approach to study pericytes in a cell-specific manner.
“We used laser light like a switch to turn individual pericytes on. This was done by combining two-photon microscopy and optogenetics, something that has been done for years to stimulate neurons, but has only recently been applied to other cell types like pericytes,” said Hartmann, a MD/PhD student who worked in Shih’s lab and is now a medical intern at Stanford University. “The important thing is we could manipulate just one pericyte at a time to understand their specific role in blood flow regulation. Previous studies were unable to flip just one light switch, they turned on the entire circuit breaker.”
Their methods involved first engineering animal models with pericytes that could be stimulated with light. Then, they captured the capillaries’ response to stimulation using real-time imaging of the living brain.
“When we turned on pericytes, we observed a direct effect of decreased capillary blood flow,” Hartmann said. “When we removed pericytes by ablating them with focused light, we observed an increase in capillary blood flow.”
The findings confirmed that brain capillary pericytes in adult animal models do in fact regulate blood flow, with one key difference: it happens much more slowly than what is observed with upstream arteries arteries.
“Blood flow changes in arteries can shift within seconds whereas blood flood changes by capillary pericytes might happen over many minutes or even hours,” Shih said.
When the research team introduced a clinically used drug, fasudil, known to relax blood vessels into the models, they were unable to manipulate the pericytes in the same way.
“Fasudil acted on the pericytes and stopped them from constricting to optogenetic stimulation,” Shih said. “With this evidence, we can begin to think of different brain conditions where it may be possible to identify therapeutics able to relax the pericytes in the living brain and improve blood flow.”
Implications for stroke and beyond
Stroke is one example of the broad implications of this research. In stroke, a clot forms causing blood flow to stop. Without oxygenated blood, brain tissue is rapidly damaged.
“Research has shown following a stroke or other conditions like brain or spinal cord injury, even as blood flow in arteries returns over time, capillary pericytes remain in a contracted state for a long time, impairing the entry of blood into the brain tissue,” Shih said. “Clinical imaging will confirm normal blood flow in the big vessels, but what are the implications of impeding blood flow in these tiny vessels we can’t see and how might we use therapeutics to alleviate that resistance?”
Their findings also extend to Alzheimer’s disease, vascular dementia and other conditions of the aging brain marked by accelerated pericyte pathology. Future studies will investigate how loss of pericyte function impacts blood distribution in the brain. Other ongoing research is studying pericytes in vasculature of the developing brain.
“Now that we know more about the dynamics of pericytes in controlling blood flow in the healthy brain, we can start to look for changes to these pericyte functions during disease,” said Berthiaume, a postdoctoral researcher in the Shih lab. “In other words, now that we know how pericytes should behave to support brain health, we can work to understand what happens when things go wrong like in stroke or injury.”
Shih is also collaborating with Dr. Juliane Gust, a pediatric neurologist studying the neurological side effects of chimeric antigen receptor (CAR) T cell cancer immunotherapies. Studies have suggested pericytes express the same antigen the CAR T cells are programmed to target on cancer cells, causing the engineered cells to attack them. New models are needed to study this more closely in the lab.
“There are a lot of tantalizing questions to follow up on now that we have a better understanding of what capillary pericytes do in the healthy brain and improved tools to study them in the lab,” Shih said.
About this neuroscience research news
Source: Seattle Children’s Hospital
Contact: Kathryn Mueller – Seattle Children’s Hospital
Image: The image is credited to Shih Lab, Seattle Children’s Research Institute
Original Research: Closed access.
“Brain capillary pericytes exert a substantial but slow influence on blood flow” by David A. Hartmann, Andrée-Anne Berthiaume, Roger I. Grant, Sarah A. Harrill, Tegan Koski, Taryn Tieu, Konnor P. McDowell, Anna V. Faino, Abigail L. Kelly & Andy Y. Shih. Nature Neuroscience
Abstract
Brain capillary pericytes exert a substantial but slow influence on blood flow
The majority of the brain’s vasculature is composed of intricate capillary networks lined by capillary pericytes. However, it remains unclear whether capillary pericytes influence blood flow.
Using two-photon microscopy to observe and manipulate brain capillary pericytes in vivo, we find that their optogenetic stimulation decreases lumen diameter and blood flow, but with slower kinetics than similar stimulation of mural cells on upstream pial and precapillary arterioles. This slow vasoconstriction was inhibited by the clinically used vasodilator fasudil, a Rho-kinase inhibitor that blocks contractile machinery.
Capillary pericytes were also slower to constrict back to baseline following hypercapnia-induced dilation, and slower to dilate towards baseline following optogenetically induced vasoconstriction. Optical ablation of single capillary pericytes led to sustained local dilation and a doubling of blood cell flux selectively in capillaries lacking pericyte contact.
These data indicate that capillary pericytes contribute to basal blood flow resistance and slow modulation of blood flow throughout the brain.






