Summary: A rare autoimmune disorder popularized by the autobiography and movie “Brain on Fire” is triggered by an attack on NMDA receptors. The disease occurs when antibodies attack NMDA receptors in the brain, leading to memory loss, intellectual changes, seizures, and death.
Source: Oregon Health & Science University
Scientists have developed an animal model that may provide a path toward improving the diagnosis and treatment of the devastating brain disease chronicled in the bestselling autobiography “Brain on Fire.” The book, along with a 2017 movie by the same name, traces newspaper reporter Susannah Cahalan’s harrowing descent into the throes of the disease.
The autoimmune disease is triggered by an attack on one of the key neurotransmitter receptors in the brain, the NMDA receptor. The disease is characterized by intellectual changes, severe memory loss, seizures, and even death. The study, published today in the journal Science Translational Medicine, reports an original animal model for this puzzling disease.
Scientists with the Vollum Institute at OHSU in Portland, Oregon, say the discovery clears the way for developing a near-atomic image of how the immune system attacks the NMDA receptor. With that information, researchers could specifically block the damaging autoimmune reaction and test new therapeutic strategies using their newly developed model for the disease in mice.
“The goal is a cure that is specific to this disease,” said senior author Gary Westbrook, M.D., a senior scientist in the Vollum Institute.
Brian Jones, a doctoral student in OHSU’s neuroscience graduate program, and Westbrook collaborated with scientists in the lab of co-author Eric Gouaux, Ph.D. In 2014, Gouaux, also a senior scientist in the Vollum and investigator with the Howard Hughes Medical Institute, published an unprecedented three-dimensional view of the NMDA receptor in the journal Nature.
NMDA receptors are present in almost every neuron in the brain. Crucial for learning and memory, these receptors are required for the formation of new memories in the hippocampus of humans and animals and have been implicated in several neurological and psychiatric conditions including Alzheimer’s disease, schizophrenia, and depression.
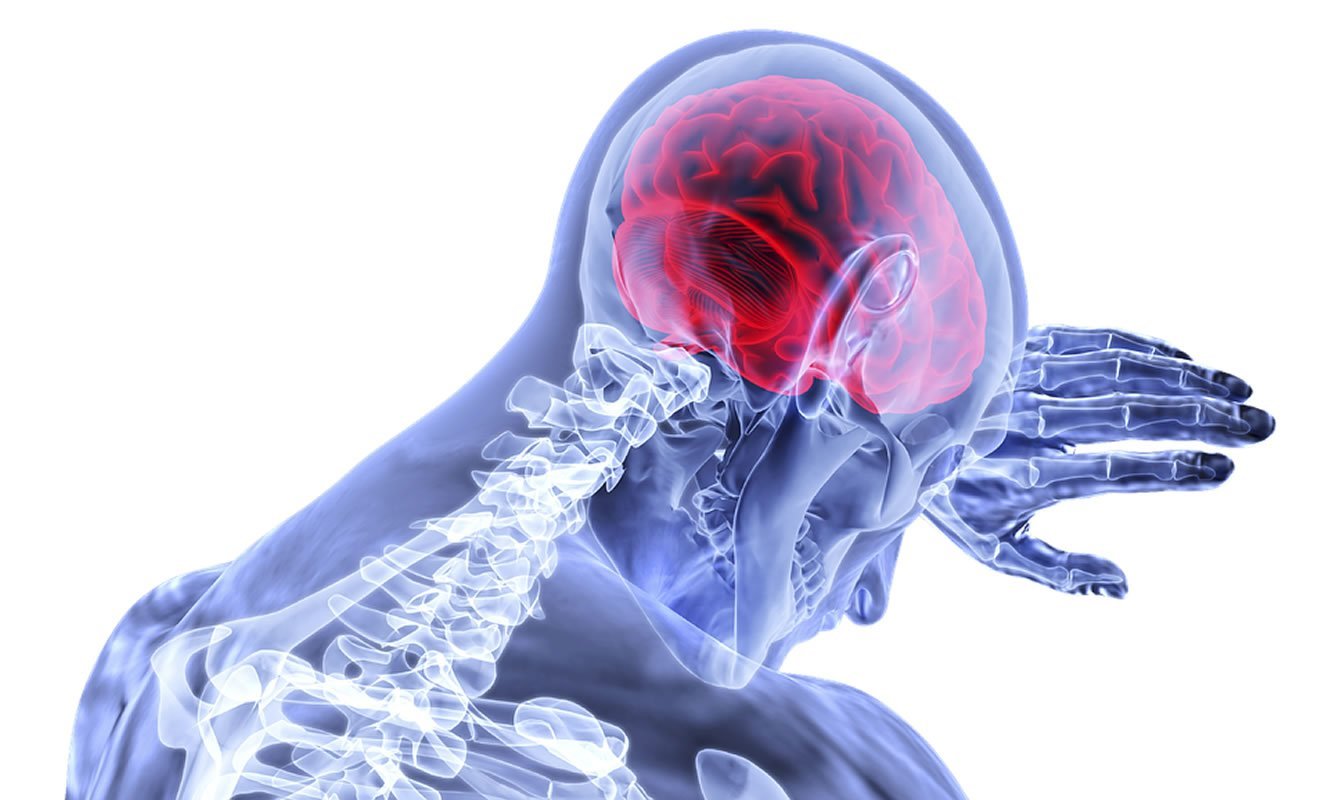
The disease in people occurs unexpectedly when antibodies produced by the body’s own immune system attack NMDA receptors in the brain.
“It’s a human disease that we don’t know enough about,” Westbrook said.
Westbrook, also the Dixon professor of neurology in the OHSU School of Medicine, said the animal model will be especially useful in determining the distinct site or sites on the NMDA receptor that trigger disease.
“If we know the exact site, one could potentially intervene early to abort the progression of the disease,” Westbrook said.
Westbrook’s collaboration with Gouaux’s team started three years ago when they developed a strategy to model the disease by active immunization of mice with the fully formed NMDA receptor. Almost 90% of mice developed signs that mimicked the human disease.
According to the research team, the development of an animal model is important because there are few samples of brain tissue from people who have developed the disease. The animal model provides a unique opportunity to recognize early signs of the disease and to develop therapeutic approaches that could precisely target the site on the receptor, known as an epitope, that is attacked by the immune system.
Funding: This work was supported by the National Institute of Neurological Disorders and Stroke, a branch of the National Institutes of Health, (P30NS061800) and NS038631.
Source:
Oregon Health & Science University
Media Contacts:
Erik Robinson – Oregon Health & Science University
Image Source:
The image is in the public domain.
Original Research: Closed access
“Autoimmune receptor encephalitis in mice induced by active immunization with conformationally stabilized holoreceptors”. April Goehring, et al.
Science Translational Medicine. doi:10.1126/scitranslmed.aaw0044
Abstract
Autoimmune receptor encephalitis in mice induced by active immunization with conformationally stabilized holoreceptors
Autoimmunity to membrane proteins in the central nervous system has been increasingly recognized as a cause of neuropsychiatric disease. A key recent development was the discovery of autoantibodies to N-methyl-D-aspartate (NMDA) receptors in some cases of encephalitis, characterized by cognitive changes, memory loss, and seizures that could lead to long-term morbidity or mortality. Treatment approaches and experimental studies have largely focused on the pathogenic role of these autoantibodies. Passive antibody transfer to mice has provided useful insights but does not produce the full spectrum of the human disease. Here, we describe a de novo autoimmune mouse model of anti-NMDA receptor encephalitis. Active immunization of immunocompetent mice with conformationally stabilized, native-like NMDA receptors induced a fulminant encephalitis, consistent with the behavioral and pathologic characteristics of human cases. Our results provide evidence for neuroinflammation and immune cell infiltration as components of the autoimmune response in mice. Use of transgenic mice indicated that mature T cells and antibody-producing cells were required for disease induction. This active immunization model may provide insights into disease induction and a platform for testing therapeutic approaches.






