Summary: Capillary constriction caused by amyloid beta restricts cerebral blood flow in mouse models of Alzheimer’s disease. Inhibiting the capillary constriction may potentially reduce some of the neurodegenerative features of Alzheimer’s.
Source: UCL
Reduced blood flow to the brain associated with early Alzheimer’s may be caused by the contraction of cells wrapped around blood vessels, according to a UCL-led study that opens up a new way to potentially treat the disease.
Blood provides the brain’s energy supply in the form of glucose and oxygen. Earlier studies have suggested the first change in Alzheimer’s disease is a decrease in cerebral blood flow.
The study, published today in the journal Science and funded by the European Research Council, Wellcome and the Wolfson Foundation, looked at the role of pericytes, cells wrapped around capillaries that have the ability to contract and regulate blood flow.
Researchers examined capillaries in Alzheimer’s-affected human brain tissue and in mice bred to develop Alzheimer’s pathology, and found that they were squeezed by pericytes. They also applied amyloid beta protein (which accumulates in the brains of people with Alzheimer’s) to slices of healthy brain tissue, and found that the capillaries were squeezed as a result.
They calculated that the constriction was severe enough to halve blood flow, which is comparable to the decrease in blood flow found in parts of the brain affected by Alzheimer’s.
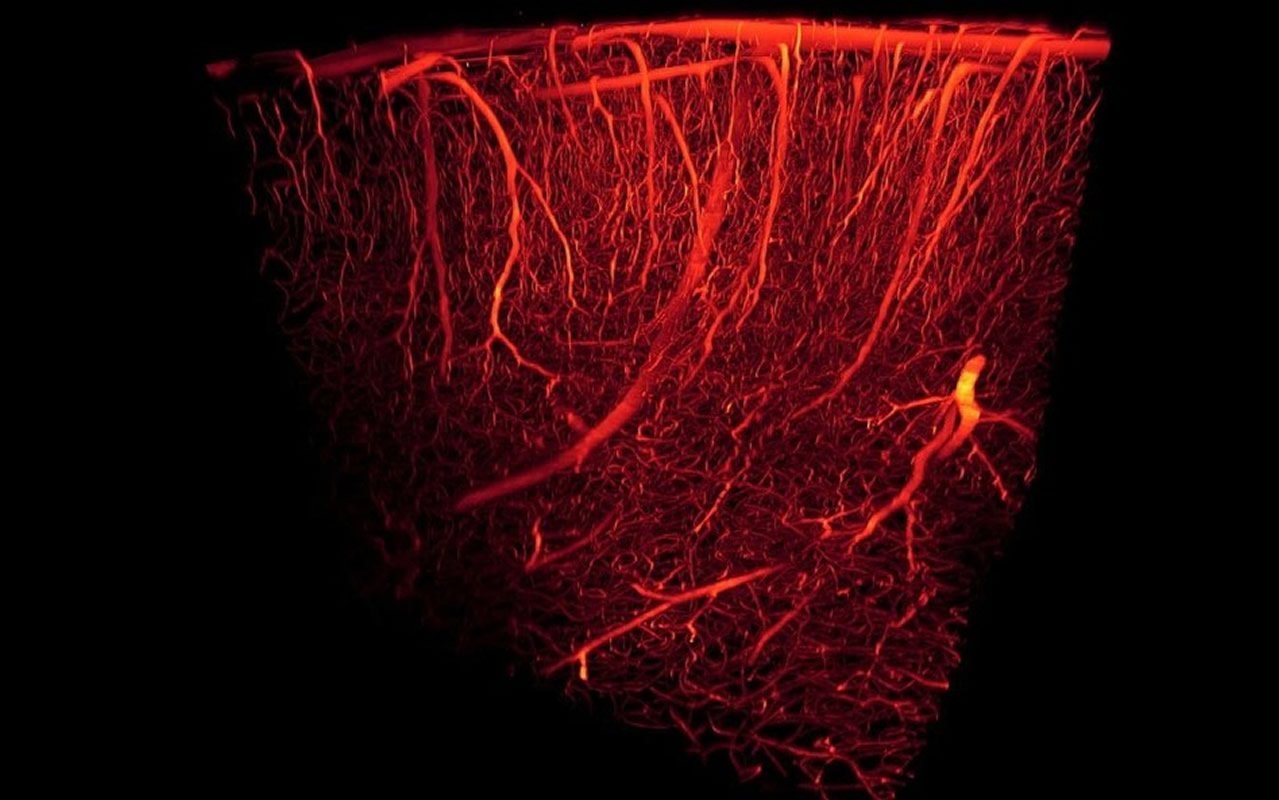
Lead author Dr Ross Nortley (UCL Queen Square Institute of Neurology) said: “Our study has, for the first time, identified the underlying mechanism behind the reduction of brain blood flow in Alzheimer’s disease.
“Since reduced blood flow is the first clinically detectable sign of Alzheimer’s, our research generates new leads for possible treatments in the early phase of the disease.”
Professor David Attwell (UCL Neuroscience, Physiology & Pharmacology), senior author of the study, said: “Damage to synapses and neurons in Alzheimer’s is usually attributed to the actions of amyloid and tau proteins accumulating in the brain.
“Our research raises the question of what fraction of the damage is a consequence of the decrease in energy supply that amyloid produces by constricting the brain’s finer blood vessels.”
“In clinical trials, drugs that clear amyloid beta from the brain have not succeeded in slowing mental decline at a relatively late phase of the disease. We now have a new avenue for therapies intervening at an earlier stage.”
The finding raises the prospect of treatments for Alzheimer’s that are focused on keeping the pericytes relaxed.
Source:
UCL
Media Contacts:
Mark Greaves – UCL
Image Source:
The image is credited to Antonino Paolo Di Giovanna.
Original Research: Closed access
“Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes”. David Attwell et al.
Science. doi:10.1126/science.aav9518
Abstract
Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes
Cerebral blood flow is reduced early in Alzheimer’s disease (AD). Because most of the vascular resistance within the brain is in capillaries, this could reflect dysfunction of contractile pericytes on capillary walls. Here we used live and rapidly-fixed biopsied human tissue to establish disease-relevance, and rodent experiments to define mechanism. We found that, in humans with cognitive decline, amyloid β (Aβ) constricts brain capillaries at pericyte locations. This was caused by Aβ generating reactive oxygen species, which evoked the release of endothelin-1 (ET) that activated pericyte ETA receptors. Capillary, but not arteriole, constriction also occurred in vivo in a mouse model of AD. Thus, inhibiting the capillary constriction caused by Aβ could potentially reduce energy lack and neurodegeneration in AD.






