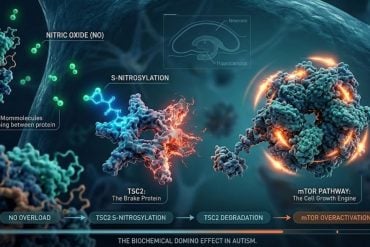
Summary: Alzheimer’s disease (AD) progression varies based on the presence of tau and amyloid-beta (Aβ) proteins in the brain. Patients with high levels of both tau and Aβ experience rapid memory decline, while those with high Aβ but low tau show a slower progression.
The research emphasizes that tau levels are crucial for diagnosing and managing AD effectively. This insight could lead to more personalized treatment strategies as biomarker technology advances.
Key Facts:
- High tau and Aβ levels lead to faster cognitive decline in Alzheimer’s patients.
- Patients with high Aβ but low tau have a slower progression of memory loss.
- Measuring tau and Aβ could help tailor treatment plans for Alzheimer’s disease.
Source: Karolinska Institute
A study from Karolinska Institutet, published in the journal Molecular Psychiatry, offers new insights into the progression of Alzheimer’s disease (AD). The research reveals critical differences in the progression of memory loss based on the presence of specific proteins in the brain.
Alzheimer’s disease is the most common form of dementia, characterized by the accumulation of amyloid-beta (Aβ) and tau proteins in the brain, leading to cognitive decline.
Traditionally, memory clinics have relied on positron emission tomography (PET) to detect Aβ accumulation. However, recent advances now allow for the detection of tau protein accumulation as well.
The study highlights that individuals with symptoms of memory loss and high levels of tau protein (tau positivity) also exhibit high levels of Aβ (Aβ positivity). This combination is associated with a malignant progression of memory loss due to AD. However, individuals who are Aβ positive but tau negative may experience a more benign progression of symptoms.
“If an individual is positive for Aβ but negative for tau, then the progression of symptoms is likely to be benign. If the symptoms are malignant, it is possible that the cause is not exclusively AD but may also involve other medical conditions that contribute to memory loss.
“If both Aβ and tau are absent, the cause of memory loss is likely another medical condition rather than AD,” explains Konstantinos Ioannou, Ph.D. student at the Department of Neurobiology, Care Sciences and Society.
This distinction is crucial for accurate diagnosis and treatment planning.
The presence of high levels of tau protein is strongly linked to cognitive decline due to AD. For individuals with high Aβ levels but low tau levels, memory loss may result from multiple medical conditions. In the era of emerging drugs that can remove Aβ, identifying the primary cause of memory loss is essential for determining the most effective treatment for each patient.
“Developing biomarker panels that measure multiple proteins simultaneously will help us understand the complexity of each patient,” says Ioannou, first author of the study. “This approach will allow for more personalized management, tailored to the prognosis or treatment of other contributing factors.”
The research team used PET to measure Aβ and tau protein levels, as well as brain function, in both healthy individuals and patients. Statistical modeling was then applied to evaluate memory loss progression across all participants. Finally, the medical histories of patients with varying levels of tau protein were compared.
Looking ahead, the researchers plan to measure Aβ and tau levels in cerebrospinal fluid and blood samples. This could potentially allow for easier clinical testing to identify individuals for whom AD is the primary cause of memory loss.
Additional studies, including MRI scans and brain data from deceased individuals, are also planned to confirm these findings before introducing tau protein measurement into clinical practice.
This study marks a significant step forward in understanding Alzheimer’s disease and improving patient care through more precise diagnostic tools and personalized treatment plans.
About this Alzheimer’s disease and memory research news
Author: Konstantinos Ioannou
Source: Karolinska Institute
Contact: Konstantinos Ioannou – Karolinska Institute
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Tau PET positivity predicts clinically relevant cognitive decline driven by Alzheimer’s disease compared to comorbid cases; proof of concept in the ADNI study” by Konstantinos Ioannou et al. Molecular Psychiatry
Abstract
Tau PET positivity predicts clinically relevant cognitive decline driven by Alzheimer’s disease compared to comorbid cases; proof of concept in the ADNI study
β-amyloid (Aβ) pathology is not always coupled with Alzheimer’s disease (AD) relevant cognitive decline.
We assessed the accuracy of tau PET to identify Aβ(+) individuals who show prospective disease progression. 396 cognitively unimpaired and impaired individuals with baseline Aβ and tau PET and a follow-up of ≥ 2 years were selected from the Alzheimer’s Disease Neuroimaging Initiative dataset.
The participants were dichotomously grouped based on either clinical conversion (i.e., change of diagnosis) or cognitive deterioration (fast (FDs) vs. slow decliners (SDs)) using data-driven clustering of the individual annual rates of cognitive decline.
To assess cognitive decline in individuals with isolated Aβ(+) or absence of both Aβ and tau (T) pathologies, we investigated the prevalence of non-AD comorbidities and FDG PET hypometabolism patterns suggestive of AD.
Baseline tau PET uptake was higher in Aβ(+)FDs than in Aβ(-)FD/SDs and Aβ(+)SDs, independently of baseline cognitive status. Baseline tau PET uptake identified MCI Aβ(+) Converters and Aβ(+)FDs with an area under the curve of 0.85 and 0.87 (composite temporal region of interest) respectively, and was linearly related to the annual rate of cognitive decline in Aβ(+) individuals.
The T(+) individuals constituted largely a subgroup of those being Aβ(+) and those clustered as FDs. The most common biomarker profiles in FDs (n = 70) were Aβ(+)T(+) (n = 34, 49%) and Aβ(+)T(-) (n = 19, 27%).
Baseline Aβ load was higher in Aβ(+)T(+)FDs (M = 83.03 ± 31.42CL) than in Aβ(+)T(-)FDs (M = 63.67 ± 26.75CL) (p-value = 0.038). Depression diagnosis was more prevalent in Aβ(+)T(-)FDs compared to Aβ(+)T(+)FDs (47% vs. 15%, p-value = 0.021), as were FDG PET hypometabolism pattern not suggestive of AD (86% vs. 50%, p-value = 0.039).
Our findings suggest that high tau PET uptake is coupled with both Aβ pathology and accelerated cognitive decline. In cases of isolated Aβ(+), cognitive decline may be associated with changes within the AD spectrum in a multi-morbidity context, i.e., mixed AD.