Summary: Researchers leveraged cutting-edge technology to gain insights into schizophrenia’s neurodevelopmental origins. The researchers grew brain organoids from patients’ skin cells, finding persistent axonal disruptions in those with schizophrenia.
In another study, researchers zeroed in on a schizophrenia risk gene, CYFIP1, revealing its potential role in brain immune cells called microglia and their influence on synaptic pruning – a crucial process for brain health.
Key Facts:
- Using brain organoids, researchers identified persistent axonal dysregulation in schizophrenia patients.
- A gene, CYFIP1, situated in a schizophrenia risk locus, affects the behavior and function of microglia, linking it to neurodevelopmental disorders.
- These studies emphasize the importance of non-neuronal cells in understanding and potentially treating neuropsychiatric disorders.
Source: Elsevier
Schizophrenia is a severe neuropsychiatric disease that remains poorly understood and treated. Schizophrenia onset is typically in adolescence or early adulthood, but its underlying causes are thought to involve neurodevelopmental abnormalities.
Because human prenatal and postnatal brain tissue is exceedingly difficult to procure and therefore study, researchers have had limited opportunities to identify early disease mechanisms, especially during the critical prenatal period.
Now, a pair of studies that appear in Biological Psychiatry use new technology to study schizophrenia in models of early human brain development.
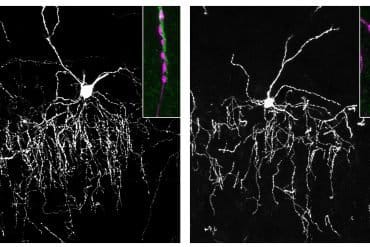
The first study used a unique approach involving three-dimensional brain organoids, which are known to recapitulate fetal brain development.
The researchers, led by first author Ibrahim A. Akkouh, PhD, and senior author Srdjan Djurovic, PhD, both at Oslo University Hospital, collected skin cells from 14 patients with schizophrenia and 14 healthy controls and generated induced pluripotent stem cells (iPSCs), which they then manipulated to develop into brain-like cortical spheroids.
The organoids grown from patients and controls differed in their expression of thousands of genes – in line with the finding that the genetic influences on schizophrenia are many and very small. However, among the genes, those associated with neuronal axons stood out as a group.
Dr. Akkouh explained, “We identified persistent axonal dysregulation as an early contribution to disease risk.”
Importantly, the researchers assessed organoid maturation at several time points, which enabled them to establish the persistent nature of the disturbances throughout development.
Dr. Akkouh added, “Our findings provide novel and hitherto inaccessible insights into the molecular basis of schizophrenia during early brain development.”
In the second study, researchers led by Roy H. Perlis, PhD, at Harvard Medical School, focused on a particular genetic risk locus. The schizophrenia risk locus 15q11.2, a particular chromosomal region containing four genes, has a penetrance of over 10%, translating to a doubling of risk for schizophrenia among people carrying an unusual copy number of this genetic region.
One gene in the locus, CYFIP1, has been associated with synaptic function in neurons and confers increased risk for neurodevelopmental disorders including schizophrenia and autism.
CYFIP1 is highly expressed in microglia, the brain’s own immune cells, but its function there is unknown. Microglia are known to carry out synaptic pruning, in which they “eat” excess synaptic structures, a process critical to healthy brain development.
Dr. Perlis and colleagues collected blood cells from healthy volunteers and isolated iPSCs, which they then manipulated to differentiate into microglia-like cells. The researchers then used CRISPR technology to remove functional CYFIP1 from the cells.
Dr. Perlis said of the work, “Our findings suggest that changes in the behavior and function of microglia due to aberrant CYFIP1 function, such as through coding or copy number variants, could affect microglial processes such as synaptic pruning, homeostatic surveillance, and neuronal maintenance, which are critical for proper brain development and function.
“This could contribute to CYFIP1-related neurodevelopmental and psychiatric disorders resulting in part from microglia dysfunction. Among the specific disorders linked to variation in CYFIP1 are both autism and schizophrenia.”
John Krystal, MD, Editor of Biological Psychiatry, commented, “The biology of schizophrenia is very complex and yet two themes represented by these two studies seem to be very important: the increased rate of elimination of glutamatergic synapses during development, and disturbances in the signaling properties of these glutamate synapses. These two disturbances could perturb circuit function in ways that are critical to development of symptoms and cognitive impairments associated with schizophrenia.”
Dr. Perlis added, “More broadly, our findings highlight the importance of looking beyond neurons to understand risk genes. While finding risk loci may be the first step in understanding the role of genes in brain diseases, it’s only a first step; figuring out the relevant cell type, and what those genes are doing, is absolutely critical in moving from association to – we hope – actual treatments.”
About this schizophrenia research news
Author: Eileen Leahy
Source; Elsevier
Contact: Eileen Leahy – Elsevier
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Longitudinal Transcriptomic Analysis of Human Cortical Spheroids Identifies Axonal Dysregulation in the Prenatal Brain as a Mediator of Genetic Risk for Schizophrenia” by Srdjan Djurovic et al. Biological Psychiatry
Open access.
“Loss of Function in the Neurodevelopmental Disease and Schizophrenia-Associated Gene CYFIP1 in Human Microglia-like Cells Supports a Functional Role in Synaptic Engulfment” by Roy Perlis et al. Biological Psychiatry
Abstract
Longitudinal Transcriptomic Analysis of Human Cortical Spheroids Identifies Axonal Dysregulation in the Prenatal Brain as a Mediator of Genetic Risk for Schizophrenia
Background
Schizophrenia (SCZ) has a known neurodevelopmental etiology, but limited access to human prenatal brain tissue hampers the investigation of basic disease mechanisms in early brain development. Here, we elucidate the molecular mechanisms contributing to SCZ risk in a disease-relevant model of the prenatal human brain.
Methods
We generated induced pluripotent stem cell–derived organoids, termed human cortical spheroids (hCSs), from a large, genetically stratified sample of 14 SCZ cases and 14 age- and sex-matched controls. The hCSs were differentiated for 150 days, and comprehensive molecular characterization across 4 time points was carried out.
Results
The transcriptional and cellular architecture of hCSs closely resembled that of fetal brain tissue at 10 to 24 postconception weeks, showing strongest spatial overlap with frontal regions of the cerebral cortex. A total of 3520 genes were differentially modulated between SCZ and control hCSs across organoid maturation, displaying a significant contribution of genetic loading, an overrepresentation of risk genes for autism spectrum disorder and SCZ, and the strongest enrichment for axonal processes in all hCS stages. The two axon guidance genes SEMA7A and SEMA5A, the first a promoter of synaptic functions and the second a repressor, were downregulated and upregulated, respectively, in SCZ hCSs. This expression pattern was confirmed at the protein level and replicated in a large postmortem sample.
Conclusions
Applying a disease-relevant model of the developing fetal brain, we identified consistent dysregulation of axonal genes as an early risk factor for SCZ, providing novel insights into the effects of genetic predisposition on the neurodevelopmental origins of the disorder.
Abstract
Loss of Function in the Neurodevelopmental Disease and Schizophrenia-Associated Gene CYFIP1 in Human Microglia-like Cells Supports a Functional Role in Synaptic Engulfment
Background
The CYFIP1 gene, located in the neurodevelopmental risk locus 15q11.2, is highly expressed in microglia, but its role in human microglial function as it relates to neurodevelopment is not well understood.
Methods
We generated multiple CRISPR (clustered regularly interspaced short palindromic repeat) knockouts of CYFIP1 in patient-derived models of microglia to characterize function and phenotype. Using microglia-like cells reprogrammed from peripheral blood mononuclear cells, we quantified phagocytosis of synaptosomes (isolated and purified synaptic vesicles) from human induced pluripotent stem cell (iPSC)–derived neuronal cultures as an in vitro model of synaptic pruning. We repeated these analyses in human iPSC-derived microglia-like cells derived from 3 isogenic wild-type/knockout line pairs derived from 2 donors and further characterized microglial development and function through morphology and motility.
Results
CYFIP1 knockout using orthogonal CRISPR constructs in multiple patient-derived cell lines was associated with a statistically significant decrease in synaptic vesicle phagocytosis in microglia-like cell models derived from both peripheral blood mononuclear cells and iPSCs. Morphology was also shifted toward a more ramified profile, and motility was significantly reduced. However, iPSC-CYFIP1 knockout lines retained the ability to differentiate to functional microglia.
Conclusions
The changes in microglial phenotype and function due to the loss of function of CYFIP1 observed in this study implicate a potential impact on processes such as synaptic pruning that may contribute to CYFIP1-related neurodevelopmental disorders. Investigating risk genes in a range of central nervous system cell types, not solely neurons, may be required to fully understand the way in which common and rare variants intersect to yield neuropsychiatric disorders.