Summary: Study reveals the role the mouse gene Ophn1 plays in helpless behaviors and identified three methods in which to reverse the effect.
Source: Cold Spring Harbor Laboratory
Everyone faces stress occasionally, whether in school, at work, or during a global pandemic. However, some cannot cope as well as others. In a few cases, the cause is genetic. In humans, mutations in the OPHN1 gene cause a rare X-linked disease that includes poor stress tolerance.
Cold Spring Harbor Laboratory (CSHL) Professor Linda Van Aelst seeks to understand factors that cause specific individuals to respond poorly to stress. She and her lab studied the mouse gene Ophn1, an analog of the human gene, which plays a critical role in developing brain cell connections, memories, and stress tolerance.
When Ophn1 was removed in a specific part of the brain, mice expressed depression-like helpless behaviors. The researchers found three ways to reverse this effect.
To test for stress, the researchers put mice into a two-room cage with a door in between. Normal mice escape from the room that gives them a light shock on their feet. But animals lacking Ophn1 sit helplessly in that room without trying to leave. Van Aelst wanted to figure out why.
Her lab developed a way to delete the Ophn1 gene in different brain regions. They found that removing Ophn1 from the prelimbic region of the medial prefrontal cortex (mPFC), an area known to influence behavioral responses and emotion, induced the helpless phenotype. Then the team figured out which brain circuit was disrupted by deleting Ophn1, creating overactivity in the brain region and ultimately the helpless phenotype.
Understanding the circuit
Pyramidal neurons are central to this brain circuit. If they fire too much, the mouse becomes helpless.
Another cell, an interneuron, regulates the pyramidal neuron activity, making sure it does not fire too much.
These two cells feedback to each other, creating a loop.
Ophn1 controls a particular protein, RhoA kinase, within this feedback loop which helps regulate and balances activity.
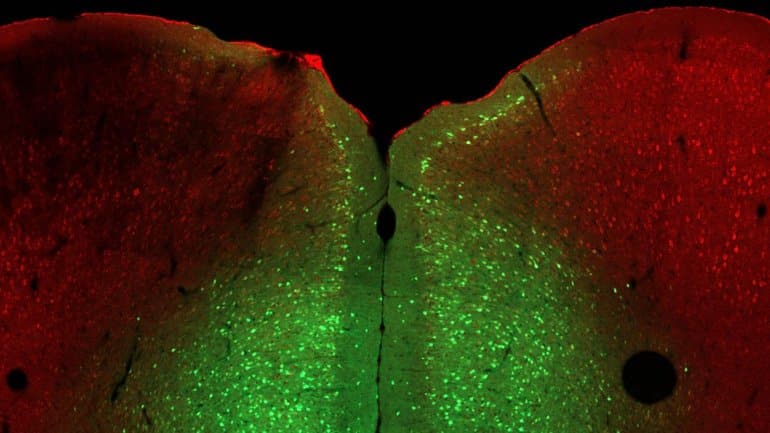
Van Aelst found three agents that reversed the helpless phenotype. Fasudil, an inhibitor specific for RhoA kinase, mimicked the effect of the missing Ophn1. A second drug dampens excess pyramidal neuron activity. A third drug wakes up the interneurons to inhibit pyramidal neurons. Van Aelst says:
“So bottom line, if you can restore the proper activity in the medial prefrontal cortex, then you could rescue the phenotype. So that was actually very exciting. You should be open to anything. You never know. Everything is surprising.”
Van Aelst hopes that understanding the complex feedback loop behind Ophn1-related stress responses will lead to better treatments for stress in humans.
About this genetics and stress research news
Source: Cold Spring Harbor Laboratory
Contact: Sara Roncero-Menendez – Cold Spring Harbor Laboratory
Image: The image is credited to Minghui Wang/Van Aelst Lab, CSHL/2021
Original Research: Closed access.
“Oligophrenin-1 moderates behavioral responses to stress by regulating parvalbumin interneuron activity in the medial prefrontal cortex” by Linda Van Aelst et al. Neuron
Abstract
Oligophrenin-1 moderates behavioral responses to stress by regulating parvalbumin interneuron activity in the medial prefrontal cortex
Highlights
- •Ophn1 deficiency markedly enhances stress-induced helpless/depressive-like behavior
- •Ophn1 deletion in PV interneurons in PL-mPFC is sufficient to induce helplessness
- •Ophn1 deficiency in PV interneurons leads to enhanced PL-mPFC neuronal activity
- •Inhibiting neuronal activity or RhoA/Rho-kinase pathway reverses helpless behavior
Summary
Ample evidence indicates that individuals with intellectual disability (ID) are at increased risk of developing stress-related behavioral problems and mood disorders, yet a mechanistic explanation for such a link remains largely elusive.
Here, we focused on characterizing the syndromic ID gene oligophrenin-1 (OPHN1). We find that Ophn1 deficiency in mice markedly enhances helpless/depressive-like behavior in the face of repeated/uncontrollable stress.
Strikingly, Ophn1 deletion exclusively in parvalbumin (PV) interneurons in the prelimbic medial prefrontal cortex (PL-mPFC) is sufficient to induce helplessness. This behavioral phenotype is mediated by a diminished excitatory drive onto Ophn1-deficient PL-mPFC PV interneurons, leading to hyperactivity in this region. Importantly, suppressing neuronal activity or RhoA/Rho-kinase signaling in the PL-mPFC reverses helpless behavior.
Our results identify OPHN1 as a critical regulator of adaptive behavioral responses to stress and shed light onto the mechanistic links among OPHN1 genetic deficits, mPFC circuit dysfunction, and abnormalities in stress-related behaviors.