Summary: The mysterious workings of tingible body macrophages (TBMs) have been substantially demystified. These TBMs, a subtype of macrophages within our lymph nodes, were found to devour antibody-producing B cells, a function essential in defending our bodies against harmful pathogens.
Researchers utilized genetically engineered mice and advanced microscopy to observe the TBMs’ eating habits and diet, illuminating their essential role in preventing the production of harmful antibodies. This discovery may offer a key to the treatment of currently incurable autoimmune diseases.
Key Facts:
- Tingible body macrophages (TBMs) are identified to devour B cells, a function crucial for immunity and preventing harmful antibodies from forming.
- The study conducted on genetically engineered mice revealed that TBMs preferentially target mutating B cells within the lymph nodes, indicating a selective and strategic function.
- The origin of TBMs has been traced to the bone marrow, and the timing of their arrival in the lymph nodes is influenced by B cell training, underscoring their role in the clean-up process after an immune response.
Source: Weizmann Institute of Science
Parents tell their children to eat all the food on their plate, down to the last crumb. Certain cells within our lymph nodes, like obedient children, diligently follow this instruction.
Although these cells – a subtype of macrophages, or “big eaters” – were first described in 1884 by German biologist Walther Flemming, their origin and mode of operation had until now remained a mystery.
In a study published in the Journal of Experimental Medicine, a team of researchers headed by Prof. Ziv Shulman of the Weizmann Institute of Science has made discoveries that largely demystify the tingible body macrophages (TBMs).
These tingible (i.e., stainable) cells turn out to eat every last morsel of another type of immune cell, to help protect our bodies from harm.
The cells bound for the dinner plate are the antibody-producing B cells, which reside in lymph nodes in readiness for invasion by pathogens such as viruses or bacteria. When an invasion occurs, they become active, divide rapidly and enter transient structures called germinal centers.
These centers, situated in designated niches within lymph nodes, are “training camps” in which the B cells hone their defenses against a particular enemy. The B cells undergo an accelerated evolution of sorts, experiencing random mutations a million times faster than normal cells, a process that increases the binding capacity of the antibodies they produce.
On rare occasions, however, other B cells may evolve to produce autoantibodies that target and harm the body’s own healthy tissues instead of pathogens.
By the end of the “training program,” cells that have undergone beneficial mutations survive for the most part, whereas those bearing ineffective or even harmful mutations commit “suicide” through a mechanism of programmed cell death. All these dead B cells, though inactive, can still give rise to harmful antibodies.
How is this prevented?
That’s where the “big eaters” come into play. In the new study the team, led by research student Neta Gurwicz of Weizmann’s Systems Immunology Department, used genetically engineered mice to uncover the TBMs’ eating patterns and diet.
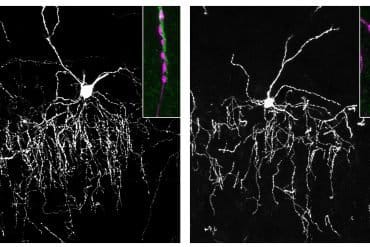
Genes for two different fluorescent proteins were used to mark the “big eaters” in shiny green and the B cells in their training camps in bright red.
As expected, a week, after the mice were vaccinated so as to activate the B cells, training camp sites opened within lymph nodes, and B cells, began to multiply, mutate and undergo selection.
Through microsurgery, the scientists exposed lymph nodes in the mice’s knees and then attached an advanced microscope with a resolution of one millionth of a meter that enabled them to observe biological processes inside a living body.
During this real-time broadcast, the team watched B cells moving constantly inside the training camp, while the “big eaters” stayed put in the vicinity, occasionally reaching out with octopus-like arms to grab dying B cells and engulf them.
The TBMs did so at a fairly rapid rate: Each TBM captured a B cell around once every 10 minutes. Using a computer model, the scientists predicted that cleaning a single training camp of all its dying cells would require about 30 TBMs.
In the live broadcast, an average of 25 TBMs were observed in each camp, indicating that the cleaning mechanism is indeed effective and thorough.
Lymph node recycle bins
Next, the researchers asked whether TBMs are choosy. Do they digest any B cell passing by, or do they prefer to snack on those within the training camps? The team discovered that when exposed to inactive B cells, TBMs did not “swallow the bait,” focusing solely on the mutating B cells.
Still, one question remained – where do TBMs come from?
To solve that riddle, the scientists replaced the mice’s immune system by first irradiating them, then introducing into their bone marrow progenitor blood cells carrying genes to color the future TBMs green.
When training camps emerged in the lymph nodes in response to the vaccination, 75 percent of the nearby TBMs were green, revealing that their origin is indeed in the bone marrow. But the green cells only appeared around those training camps a few weeks after vaccination, raising the possibility that their journey included a stopover.
The team repeated the experiment, this time shielding lymph nodes during the irradiation so that only the immune cells within these nodes would survive, while the others were eradicated.
Unlike in the previous experiment, most of the TBMs lining up for the feast were not green; they developed from cells that were already in lymph nodes prior to vaccination.
The researchers concluded that the TBMs develop from progenitor cells emerging from the bone marrow, but rather than stopping off, they go straight to lymph nodes and wait there for a while.
The scientists also discovered what causes progenitor cells to leave the bone marrow and enter lymph nodes. Five days after vaccination, when small concentrations of mutating B cells appeared in the training camps, substantial quantities of TBMs first emerged nearby.
That suggested to the researchers that the timing of TBM arrival is a function of B cell training, and that the TBMs serve the particular purpose of cleaning up after what is a highly effective, but somewhat wasteful, immune system campaign.
Finally, the scientists asked when during the process that ends in their suicide the B cells are digested by TBMs. Surprisingly, they observed B cells being engulfed alive, undergoing their final last gasps inside the TBMs.
This shows that the “big eaters” are not only “cleaning workers,” as previously thought, they also constitute a biological “recycle bin” that removes dying cells before they start breaking down, preventing harmful waste from scattering in the first place.
“Impaired TBM cleaning might cause the production of autoantibodies that would be aimed at the dead B cells but actually harm healthy tissues,” says Shulman.
“This might be one of the causes for autoimmune diseases such as lupus. Basic understanding of the origin of TBMs and their modes of action could help pave the way for autoimmune disease treatments.
“If we figure out how to make TBMs more effective at cleaning up the ‘training camps,’ we may have the key to the treatment of currently incurable diseases.”
The following researchers also participated in the study: Dr. Liat Stoler-Barak of Weizmann’s Systems Immunology Department; and Niklas Schwan, Dr. Arnab Bandyopadhyay and Prof. Michael Meyer-Hermann of Braunschweig Integrated Centre of Systems Biology, Germany.
Funding: Prof. Ziv Shulman’s research is supported by the Moross Integrated Cancer Center; the Rising Tide Foundation; the Azrieli Foundation; the Ben B. and Joyce E. Eisenberg Foundation; the Wolfson Family Charitable Trust & Wolfson Foundation; Elie Hirschfeld and Dr. Sarah Schlesinger; and Miel de Botton.
About this autoimmune disease research news
Author: Ziv Shulman
Source: Weizmann Institute of Science
Contact: Ziv Shulman – Weizmann Institute of Science
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Tingible body macrophages arise from lymph node–resident precursors and uptake B cells by dendrites” by Ziv Shulman et al. Journal of Experimental Medicine
Abstract
Tingible body macrophages arise from lymph node–resident precursors and uptake B cells by dendrites
Antibody affinity maturation depends on the formation of germinal centers (GCs) in lymph nodes.
This process generates a massive number of apoptotic B cells, which are removed by a specialized subset of phagocytes, known as tingible body macrophages (TBMs). Although defects in these cells are associated with pathological conditions, the identity of their precursors and the dynamics of dying GC B cell disposal remained unknown.
Here, we demonstrate that TBMs originate from pre-existing lymph node–resident precursors that enter the lymph node follicles in a GC-dependent manner. Intravital imaging shows that TBMs are stationary cells that selectively phagocytose GC B cells via highly dynamic protrusions and accommodate the final stages of B cell apoptosis.
Cell-specific depletion and chimeric mouse models revealed that GC B cells drive TBM formation from bone marrow–derived precursors stationed within lymphoid organs prior to the immune challenge.
Understanding TBM dynamics and function may explain the emergence of various antibody-mediated autoimmune conditions.