Summary: Researchers have uncovered a neural circuit that involves spinal neurons and a signaling pathway that is responsible for how burning pain is sensed.
Source: Case Western Reserve
The world has changed since 1664, when French philosopher and scientist Rene Descartes first claimed the brain was responsible for feeling the sensation of pain.
However, a key question remains: How exactly does the human brain feel pain? Specifically, thermal pain—like that experienced when touching an open flame or a hot pan while cooking.
A team of researchers in the neurosciences department at the Case Western Reserve University School of Medicine think they’ve found an answer—that a neural circuit involving spinal neurons and a signaling pathway––are responsible for how burning pain is sensed.
They believe their discovery, published recently in the journal Neuron, could lead to more effective treatment for chronic, pathological pain—such as shooting, stabbing and burning pain—because it may involve the same signaling pathway.
“We know that heat, cold, pressure and itching stimulations to our skin result in appropriate feelings in the brain. However, the neurons encoding the heat signals in the spinal cord were unclear,” said Hongsheng Wang, study lead author and a postdoctoral fellow at the School of Medicine.
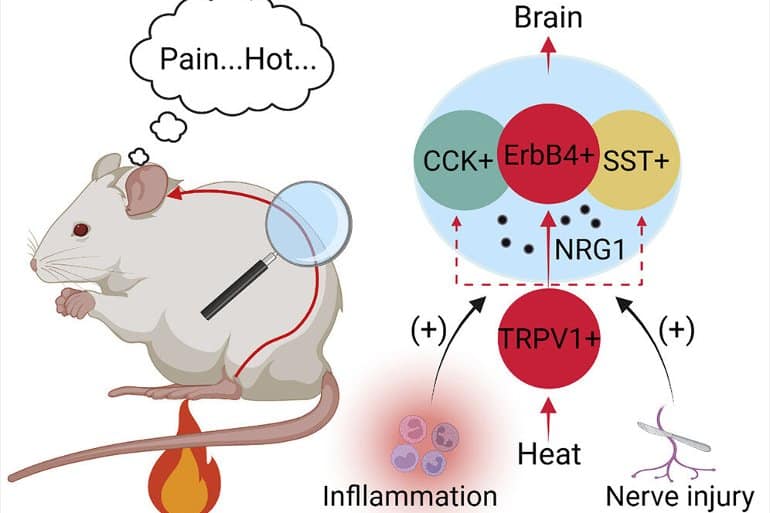
“Our study identified a group of interneurons in the spinal cord required for heat sensation. We also found a signaling pathway contributes to heat hypersensitivity caused by inflammation or nerve injuries.”
The study
The brain controls everything we do, from our perception of the world around us to how we move our bodies and experience sensations. The process involves neurons, which are cells that act as messengers to transmit information between the brain and nervous system. The neurons send information through complex circuits throughout the body.
The research team looked at neurons in the spinal cord and their role in thermal pain by analyzing mouse models and their response to heated plates. During this process, the team identified the activation of a “novel,” or newly discovered, class of spinal cord neurons (called ErbB4+) that process heat signals to the spinal cord.
The team wanted to look further into whether these neurons specifically are responsible for thermal pain. There are several ways to test this, including destroying the ErbB4+ neurons.
The researchers expressed a toxin specifically targeting the ErbB4+ neurons. Once the neurons were destroyed, the response to heat pain was impaired. This demonstrated that ErbB4+ neurons are specifically tied to how thermal pain is sensed and, when destroyed, pain is not felt less.
They also examined the role of neuregulin 1 (NRG1), a protein involved in many cellular functions. They found that NRG1 and its receptor tyrosine kinase ErbB4 (often referred to as the NRG1 signaling) is also involved in the sensation of thermal pain.
The findings
“Pain is a sensation we have all experienced. For most of us, pain is temporary,” said Lin Mei, professor and chair of the Department of Neurosciences at the School of Medicine and study corresponding author.
“However, for patients with pathological pain, the pain experience is unending, with little hope for relief. Scientists have long believed it’s a result of dysfunctional neuronal activity.”
Mei said their study showed that pathological pain can be reduced by injecting an ErbB4+ inhibitor or an NRG1 neutralizing peptide.
The application of these discoveries may go beyond the therapeutic treatment of pathological pain.
“Both NRG1 and ErbB4 are risk genes of many brain disorders including major depression and schizophrenia,” Mei said.
“Further studies are warranted to show if the mechanism of heat pain and pathological pain also plays a role in different types of pain experienced by those who have brain disorders.”
About this pain research news
Author: Press Office
Source: Case Western Reserve
Contact: Press Office – Case Western Reserve
Image: The image is credited to the researchers
Original Research: Closed access.
“A novel spinal neuron connection for heat sensation” by Hongsheng Wang et al. Neuron
Abstract
A novel spinal neuron connection for heat sensation
Highlights
- Spinal ErbB4+ neurons are activated by heat and synapsed by TRPV1+ nociceptors
- Heat sensation is reduced by ErbB4+ neuron ablation or inhibition
- Augmented effect on heat sensation by inhibiting ErbB4+, SST+, and CCK+ neurons together
- NRG1-ErbB4 signaling promotes heat sensation and hypersensitivity
Summary
Heat perception enables acute avoidance responses to prevent tissue damage and maintain body thermal homeostasis. Unlike other modalities, how heat signals are processed in the spinal cord remains unclear.
By single-cell gene profiling, we identified ErbB4, a transmembrane tyrosine kinase, as a novel marker of heat-sensitive spinal neurons in mice. Ablating spinal ErbB4+ neurons attenuates heat sensation.
These neurons receive monosynaptic inputs from TRPV1+ nociceptors and form excitatory synapses onto target neurons. Activation of ErbB4+ neurons enhances the heat response, while inhibition reduces the heat response.
We showed that heat sensation is regulated by NRG1, an activator of ErbB4, and it involves dynamic activity of the tyrosine kinase that promotes glutamatergic transmission.
Evidence indicates that the NRG1-ErbB4 signaling is also engaged in hypersensitivity of pathological pain.
Together, these results identify a spinal neuron connection consisting of ErbB4+ neurons for heat sensation and reveal a regulatory mechanism by the NRG1-ErbB4 signaling.






