Summary: Scientists have successfully reprogrammed astroglia, a type of brain support cell, into neurons that mimic specific interneurons critical for brain function. By modifying the Ascl1 protein, they increased its efficiency in converting astroglia to neuron-like cells, opening new possibilities for regenerative treatments for brain disorders such as epilepsy.
The engineered neurons exhibit high-frequency firing, a signature of certain interneurons essential for regulating brain activity. This work suggests astroglia could serve as a repair mechanism, allowing us to restore lost or damaged brain circuits.
Key Facts:
- Modified Ascl1 protein effectively converts astroglia into functional neurons.
- Reprogrammed neurons exhibit high-frequency firing, crucial for brain circuit control.
- This approach holds potential for treating conditions like epilepsy by restoring neural circuits.
Source: King’s College London
Researchers have successfully demonstrated how astroglia – cells that support the functioning of the brain – can be reprogrammed into cells resembling interneurons.
The research, published in Science Advances, represents not only an important step forward in neuronal engineering, but also has vital implications for regenerative medicine, which researchers hope could be used to restore dysfunctional brain circuits like those seen in people with epilepsy.
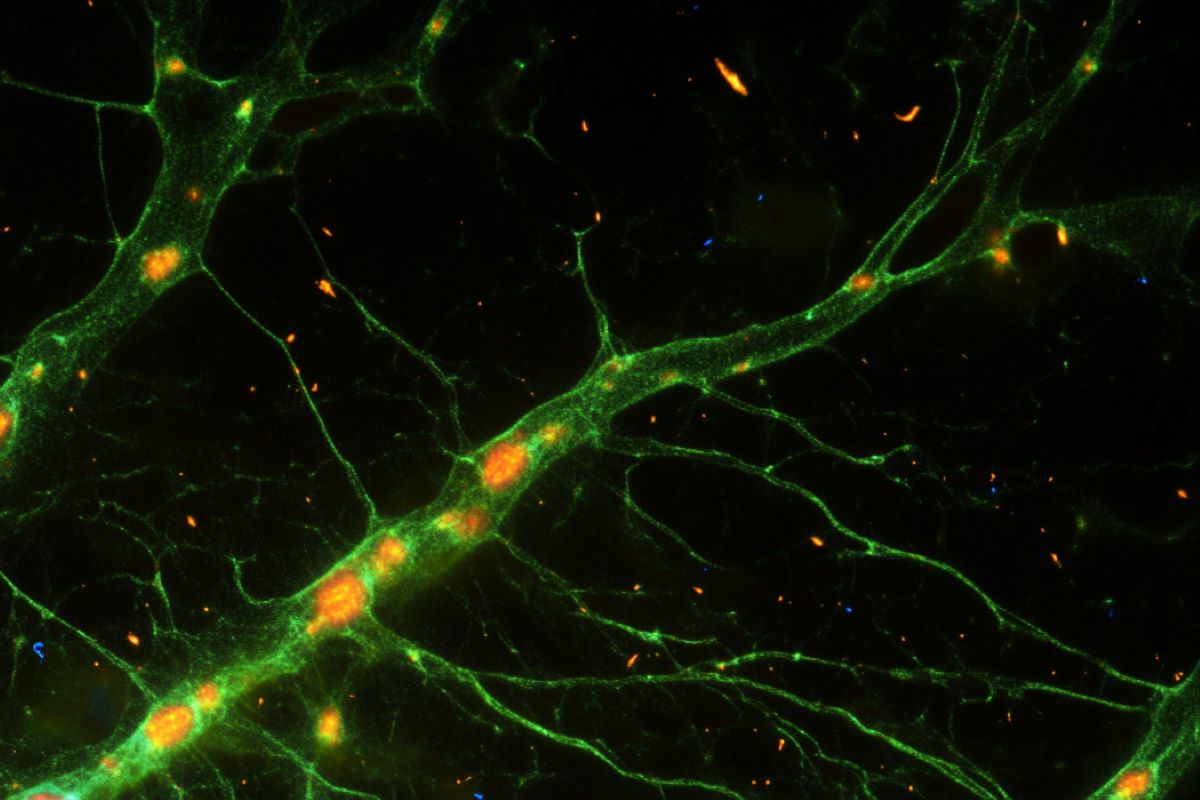
Working with mice shortly after birth, researchers coaxed astroglia to synthesize a protein, Ascl1, that plays a key role in the development of the nervous system.
They found that when mutated, Ascl1 became highly efficient in converting astroglia into functioning neurons, much more so than the form of the protein that is generated naturally by the body.
“While the neurons we induced differ from those the body creates itself, we’re excited to show that engineered neurons can acquire highly specific properties.
“Our findings will allow us to further close the gap between induced and endogenous neurons and thereby render them ever more useful for future translation in regenerative medicine.” Said Professor Benedikt Berninger, Professor of Developmental Neurobiology at King’s IoPPN and the study’s senior author
The research team found that the neurons they generated displayed properties that were similar to those native to the brains they were working on, including the ability to fire at very high frequencies, a telltale hallmark of a particular class of interneurons that play a vital role in regulating brain circuitry.
Dr Nicolás Marichal, Research Associate at the Centre for Developmental Neurobiology at King’s IoPPN and one of the study’s lead authors said, “This landmark study’s success in creating neurons from astroglia breaks new ground in regenerative medicine, offering promise for the restoration of aberrant circuitry and brain function in neurological conditions.
“This work paves the way for further research to exploit these findings and leverage lineage reprogramming of glia into subtype specific neurons as a new therapeutic avenue.”
Funding: This research was funded in part by Wellcome Trust, the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme, and the German Research Foundation.
About this neuroscience research news
Author: Benedikt Berninger
Source: King’s College London
Contact: Benedikt Berninger – King’s College London
Image: The image is credited to Neuroscience News
Original Research: Open access.
“Reprogramming astroglia into neurons with hallmarks of fast-spiking parvalbumin-positive interneurons by phospho-site–deficient Ascl1” by Benedikt Berninger et al. Science Advances
Abstract
Reprogramming astroglia into neurons with hallmarks of fast-spiking parvalbumin-positive interneurons by phospho-site–deficient Ascl1
Cellular reprogramming of mammalian glia to an induced neuronal fate holds the potential for restoring diseased brain circuits.
While the proneural factor achaete-scute complex-like 1 (Ascl1) is widely used for neuronal reprogramming, in the early postnatal mouse cortex, Ascl1 fails to induce the glia-to-neuron conversion, instead promoting the proliferation of oligodendrocyte progenitor cells (OPC).
Since Ascl1 activity is posttranslationally regulated, here, we investigated the consequences of mutating six serine phospho-acceptor sites to alanine (Ascl1SA6) on lineage reprogramming in vivo.
Ascl1SA6 exhibited increased neurogenic activity in the glia of the early postnatal mouse cortex, an effect enhanced by coexpression of B cell lymphoma 2 (Bcl2).
Genetic fate-mapping revealed that most induced neurons originated from astrocytes, while only a few derived from OPCs.
Many Ascl1SA6/Bcl2-induced neurons expressed parvalbumin and were capable of high-frequency action potential firing.
Our study demonstrates the authentic conversion of astroglia into neurons featuring subclass hallmarks of cortical interneurons, advancing our scope of engineering neuronal fates in the brain.






