Summary: Researchers have identified how a mutation causes dysfunction in a protein, which inhibits neural activity and induces migraines.
Source: CNRS.
Researchers at CNRS, Université Côte d’Azur and Inserm have demonstrated a new mechanism related to the onset of migraine. In fact, they found how a mutation, causes dysfunction in a protein which inhibits neuronal electrical activity, induces migraines. These results, published in Neuron on December 17, 2018, open a new path for the development of anti-migraine medicines.
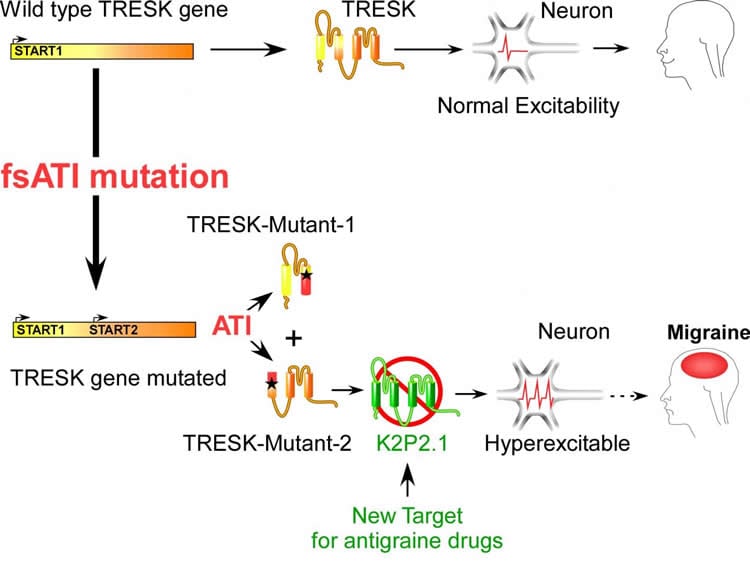
Even though 15% of the adult population worldwide suffers from migraines, no long-term, effective, curative treatment has been marketed to date. Migraine episodes are related, among other factors, to electric hyperexcitability in sensory neurons. Their electrical activity is controlled by proteins that generate current called ion channels, specifically by the TRESK channel, which inhibits electrical activity. The researchers have shown that a mutation in the gene encoding for this protein causes a split between two dysfunctional proteins: one is inactive and the other targets other ion channels (K2P2.1) inducing a great stimulation of the neuronal electrical activity causing migraines.
Though researchers had already shown the hereditary nature of migraines, they did not know the mechanism underlying migraine. By demonstrating that the TRESK split induces hyperexcitability in sensory neurons leading to migraine, this work, carried out at the Institut de Biologie Valrose (CNRS/Inserm/Université Côte d’Azur), opens new research path for the development of anti-migraine medicines. A patent application has been filed*: the scope is targeting K2P2.1 channels to reduce the electrical activity of neurons and prevent migraines from being triggered.
What is more, the researchers propose that this new genetical mechanism, causing the formation of two proteins instead of just one, has now to be considered for the study of other genetic diseases and for diagnosing them.
*Patent PCT/EP2018/067581 “Methods and compositions for treating migraine”
Source: Julie Desriac – CNRS
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Guillaume Sandoz, CNRS.
Original Research: Abstract for “Migraine-Associated TRESK Mutations Increase Neuronal Excitability through Alternative Translation Initiation and Inhibition of TREK” by Perrine Royal, Alba Andres-Bilbe, Pablo Ávalos Prado, Clément Verkest, Brigitte Wdziekonski, Sébastien Schaub, Anne Baron, Florian Lesage, Xavier Gasull, Joshua Levitz, and Guillaume Sandoz in Neuron. Published December 17 2018.
doi:10.1016/j.neuron.2018.11.039
[cbtabs][cbtab title=”MLA”]CNRS”Discovery of Novel Mechanism that Cause Migraines.” NeuroscienceNews. NeuroscienceNews, 17 December 2018.
<https://neurosciencenews.com/migraine-mechanism-10348/>.[/cbtab][cbtab title=”APA”]CNRS(2018, December 17). Discovery of Novel Mechanism that Cause Migraines. NeuroscienceNews. Retrieved December 17, 2018 from https://neurosciencenews.com/migraine-mechanism-10348/[/cbtab][cbtab title=”Chicago”]CNRS”Discovery of Novel Mechanism that Cause Migraines.” https://neurosciencenews.com/migraine-mechanism-10348/ (accessed December 17, 2018).[/cbtab][/cbtabs]
Abstract
Migraine-Associated TRESK Mutations Increase Neuronal Excitability through Alternative Translation Initiation and Inhibition of TREK
It is often unclear why some genetic mutations to a given gene contribute to neurological disorders and others do not. For instance, two mutations have previously been found to produce a dominant negative for TRESK, a two-pore-domain K+ channel implicated in migraine: TRESK-MT, a 2-bp frameshift mutation, and TRESK-C110R. Both mutants inhibit TRESK, but only TRESK-MT increases sensory neuron excitability and is linked to migraine. Here, we identify a new mechanism, termed frameshift mutation-induced alternative translation initiation (fsATI), that may explain why only TRESK-MT is associated with migraine. fsATI leads to the production of a second protein fragment, TRESK-MT2, which co-assembles with and inhibits TREK1 and TREK2, two other two-pore-domain K+ channels, to increase trigeminal sensory neuron excitability, leading to a migraine-like phenotype in rodents. These findings identify TREK1 and TREK2 as potential molecular targets in migraine and suggest that fsATI should be considered as a distinct class of mutations.






