For more than a hundred years, people have known that dendritic arbors, the projections that neurons use to receive information from other neurons, differ in size and shape depending on neuron type. Now, researchers at the RIKEN Brain Science Institute in Japan have discovered a factor helps shape dendritic arbors. Published in Nature Neuroscience, the work reveals how the protein centrosomin prevents dendrites from branching out.
Dendrites grow and branch as structural elements called microtubules push the ends out in specific directions. Microtubules are often likened to cellular scaffolding, and are built on site by growing out from one end. To determine how microtubule growth and dendritic branching is regulated, the researchers examined sensory neurons from Drosophila fruit flies.
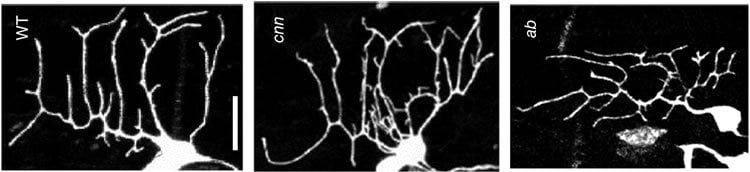
The scientists focused on a type of Drosophila sensory neuron that has very limited dendritic branching and expresses the transcription factor called Abrupt. Researchers began by determining that expression of Abrupt leads to reduced arbors, while its absence leads to more complex arbors. Next, they tested a group of candidate proteins from the pathway of molecular events initiated by Abrupt, looking for one that regulates microtubules. They found that loss of centrosomin, a protein that makes microtubule, based structures necessary for cell division, resulted in more extensive dendritic branching, and its addition could block the increase in branching caused by lack of Abrupt. The team then discovered that by working with another protein called pericentrin, centrosomin could control where new microtubules form within the dendrites.
When one end of a microtubule is attached to something, it does not push out new dendritic branches as it grows. However, when microtubules form at no particular site, the opposite is true, and new branches are more likely to form as it grows. Further testing revealed that centrosomin acts as a glue that fixes microtubules, particularly to Golgi bodies, which is why its presence promotes less complex branching.
“The shape and complexity of neuronal dendrite arbors are often disrupted in neurological diseases,” notes team leader Adrian Moore. “It turns out the two microtubule regulators we found in this study of Drosophila neurons, centrosomin and pericentrin, are encoded by genes mutated in some human brain disorders. As we learn more about how neurons control the growth of dendrites it will help us understand these diseases more completely, and we may learn how to initiate and direct neuron growth as therapy for diseases and after neuronal injury.”
Source: Adrian Walton Moore – RIKEN
Image Credit: The image is credited to the researchers/RIKEN
Original Research: Abstract for “Centrosomin represses dendrite branching by orienting microtubule nucleation” by Yalgin C, Ebrahimi S, Delandre C, Yoong LF, Akimoto S, Tran H, Amikura R, Spokony R, Torben-Nielsen B, White KP, and Moore AW in Nature Neuroscience. Published online August 31 2015 doi:10.1038/nn.4099
Abstract
Centrosomin represses dendrite branching by orienting microtubule nucleation
Neuronal dendrite branching is fundamental for building nervous systems. Branch formation is genetically encoded by transcriptional programs to create dendrite arbor morphological diversity for complex neuronal functions. In Drosophila sensory neurons, the transcription factor Abrupt represses branching via an unknown effector pathway. Targeted screening for branching-control effectors identified Centrosomin, the primary centrosome-associated protein for mitotic spindle maturation. Centrosomin repressed dendrite branch formation and was used by Abrupt to simplify arbor branching. Live imaging revealed that Centrosomin localized to the Golgi cis face and that it recruited microtubule nucleation to Golgi outposts for net retrograde microtubule polymerization away from nascent dendrite branches. Removal of Centrosomin enabled the engagement of wee Augmin activity to promote anterograde microtubule growth into the nascent branches, leading to increased branching. The findings reveal that polarized targeting of Centrosomin to Golgi outposts during elaboration of the dendrite arbor creates a local system for guiding microtubule polymerization.
“Centrosomin represses dendrite branching by orienting microtubule nucleation” by Yalgin C, Ebrahimi S, Delandre C, Yoong LF, Akimoto S, Tran H, Amikura R, Spokony R, Torben-Nielsen B, White KP, and Moore AW in Nature Neuroscience. Published online August 31 2015 doi:10.1038/nn.4099






