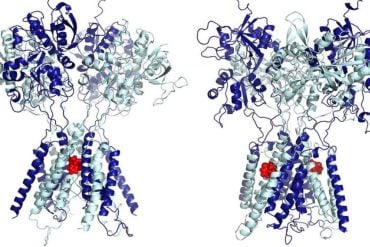
Researchers are studying how components of cell structure function in order to determine viable ways to use them for fighting such ailments as cancer, Huntington’s, Alzheimer’s, and Parkinson’s diseases.
Jianhua Xing, a Virginia Tech assistant professor of biology, and his colleagues did computational studies to compare the mechanical properties of two alternative models of the assembly of rope-like polymers called microtubules, a component of cell cytoskeletons. The researchers’ objectives are to learn how microtubules are regulated and how they assemble and disassemble.
“We want to understand the structure and regulation of microtubules because they are not static; they are always moving as they transport things in the cell,” said Xing, who also is an affiliated researcher with Fralin Research Institute. “But there are discrepancies between two models of how microtubules work that also are contradictory to experiments.”
The model of microtubule assembly that most people accept shows the individual subunits, dimers of the protein tubulin, just adding to the grow cylinder. But another model indicates that tubulin dimmers first form an open sheet structure that later closes into the cylinder. Xing and his team concluded that their computational study of the models indicate that the second model should be seriously considered for further testing.
The report of the work by Xing, Eva Nogales, and Zhanghan Wu appears in the June issue of Biophysical Journal. It’s titled “Comparative studies of microtubule mechanics with two competing models suggest function roles of alternative tubulin lateral interactions.”

Nogales, a structural biologists and Howard Hughes Medical Institute investigator at University of California, Berkeley, was instrumental in developing the latter model. She said the dynamic instability of microtubules is a crucial property for their function in cells and although much is known about microtubules, scientists still don’t understand what governs their assembly and disassembly processes.
“Our paper in Biophysical Journal is a step forward for the field, demonstrating that certain aspects in the biophysical behavior of microtubules that previously have been probed experimentally can better be explained by a new model of microtubule assembly that involves two distinct types of tubulin-tubulin lateral interactions.”
Knowing the interactions and the regulation will impact how microtubules can be used to develop drugs that could more precisely target cells involved in various diseases.
“If nature invokes the two interactions in which part of the microtubule is flattened, then it may show us the function and regulation of these structures,” Xing said.
The researchers have suggested that additional experiments should be performed on both models to determine more exactly the function and regulation of the microtubules. In the paper they state, “We believe that understanding the properties of the sheet bond holds the potential to guide the design of new tubulin-based drugs that regulate microtubule dynamics by uniquely proving that tubulin interface.”
Notes about this neurobiology research article
Wu, lead author on the paper, was a doctoral candidate in Xing’s laboratory at the time of the research. He now is a post-doctoral associate at the National Institutes of Health.
The Virginia Tech team was supported by the Thomas F. & Kate Miller Jeffress Memorial Trust and by the National Science Foundation. Eva Nogales is supported by the National Institute of General Medical Sciences and by Howard Hughes Medical Institute.
Contact: Virginia Tech news
Source: Virginia Tech (Virginia Polytechnic Institute and State University) press release
Image Source: Kinesin dimer on the microtubule image is adapted from a Wikimedia Commons image shared by user Kebes with the CC-by-SA 3.0 license.
Original Research: Abstract for “Comparative Studies of Microtubule Mechanics with Two Competing Models Suggest Functional Roles of Alternative Tubulin Lateral Interactions” by Zhanghan Wu, Eva Nogales and Jianhua Xing in Biophysical Journal 20 June 2012 Volume 102, Issue 12, 2687-2696, doi:10.1016/j.bpj.2012.05.003