Summary: Researchers have developed a dual-gene therapy approach to treat Usher syndrome type 1F, a rare condition causing deafness and progressive blindness. By splitting the large PCDH15 gene into two halves, delivering them via adeno-associated viruses, and reassembling them in cells, the therapy restored hearing and balance in mice.
Tests in human retinal organoids and primate retinas showed increased levels of protocadherin-15 in light-sensing cells, offering hope for vision preservation. This breakthrough provides a backup strategy for gene therapy and represents a step forward in addressing both hearing and vision loss in patients with Usher syndrome.
Key Facts:
- Dual-AAV therapy restored hearing and balance in Usher syndrome mouse models.
- Retinal tests suggest potential to preserve vision in humans and primates.
- The therapy offers a second strategy if earlier gene therapies prove ineffective.
Source: Harvard
Harvard Medical School researchers have taken another decisive step in their efforts to develop a gene therapy for people with Usher syndrome type 1F, a rare condition that causes deafness and progressive blindness.
A new way of delivering a corrected version of the faulty gene that causes Usher syndrome — PCDH15 — restored hearing in mouse models and showed potential in retinal organoids and nonhuman primates for improving vision, the team reports in the Journal of Clinical Investigation.
This is the second experimental gene therapy for Usher syndrome developed by the lab of David Corey, the Bertarelli Professor of Translational Medical Science in the Blavatnik Institute at HMS. Earlier research showed that a different gene-delivery strategy restored hearing in mice.
The new method provides a second option if the first approach proves unsafe or ineffective when tested in humans.
“Without a clinical trial in humans, we can’t know whether our first gene therapy restores normal function,” said Corey, senior author of the study. “This new strategy gives us a backup in case the first therapy doesn’t work. It might even turn out to be better than the first, once tested in patients.”
More strategies, more chances to cure
A central challenge in designing a therapy with the PCDH15 gene is that it’s too big to fit inside the hollowed-out shell of an adeno-associated virus, or AAV — the most common and safest vehicle used to carry genes into target cells.
The team’s earlier strategy involved trimming down PCDH15 into a mini gene that could fit in an AAV.
The new strategy entails cutting the complete gene in two, inserting each half into an AAV, and delivering the AAVs to the inner ear or eye. There, the halves rejoin and start instructing the cells to make the protein the gene codes for, protocadherin-15, correctly.
People with Usher syndrome type 1F are born without hearing or a sense of balance and gradually lose their vision. The new approach restored hearing and balance in the mice.
Existing mouse models do not experience the type of vision loss observed in Usher syndrome type 1F, so the team couldn’t study whether their vision improved.
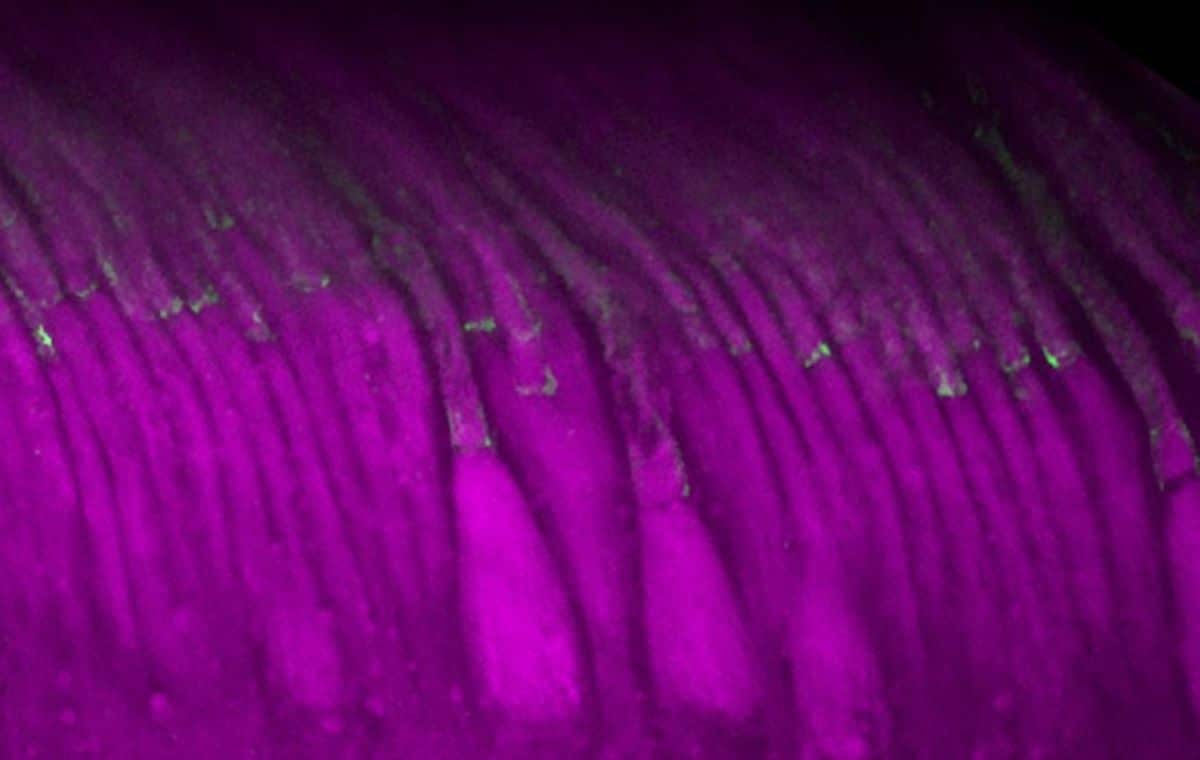
However, the dual-AAV therapy raised levels of protocadherin-15 in the light-sensing cells of human retinal organoids and nonhuman primate retinas. The protein also migrated to the right place in those cells. Both results suggest that the treatment strategy may one day act to preserve patients’ vision.
“These results are particularly exciting because, while cochlear implants can address hearing loss in human patients, there are currently no treatments for the vision dysfunction associated with Usher syndrome,” said first author Maryna Ivanchenko, HMS instructor in neurobiology in the Corey Lab and an ophthalmologist.
Authorship, funding, disclosures
Members of the Corey Lab worked with colleagues at the Institute of Molecular and Clinical Ophthalmology Basel and the University of Basel in Switzerland. Additional authors are Daniel M. Hathaway, Eric M. Mulhall, Kevin T. Booth, Mantian Wang, Cole W. Peters, Alex J. Klein, Xinlan Chen, Yaqiao Li, and Bence György.
Funding: This work was supported by the National Institutes of Health (grants R01-DC016932 and R01-DC020190 and fellowship T32-GM007748), the Bertarelli Foundation, the Seamans Family, and the Swiss National Science Foundation (grants PCEFP3_202756 and 310030_192665). The authors acknowledge the HMS MicRoN Microscopy Core and Electron Microscopy Facility and say that continued support from the Usher 1F Collaborative is especially appreciated.
The President and Fellows of Harvard College have filed U.S. Provisional Application No. 18/025,719, DUAL-AAV VECTOR DELIVERY OF PCDH15 AND USES THEREOF, with Corey, Mulhall, and Ivanchenko listed as inventors. The other authors have declared no conflict of interest.
About this genetics, vision, and auditory neuroscience research news
Author: Stephanie Dutchen
Source: Harvard
Contact: Stephanie Dutchen – Harvard
Image: The image is credited to Maryna Ivanchenko
Original Research: Open access.
“PCDH15 Dual-AAV Gene Therapy for Deafness and Blindness in Usher Syndrome Type 1F Models” by Maryna Ivanchenko et al. Journal of Clinical Investigation
Abstract
PCDH15 Dual-AAV Gene Therapy for Deafness and Blindness in Usher Syndrome Type 1F Models
Usher syndrome type 1F (USH1F), resulting from mutations in the protocadherin-15 (PCDH15) gene, is characterized by congenital lack of hearing and balance, and progressive blindness in the form of retinitis pigmentosa.
In this study, we explore an approach for USH1F gene therapy, exceeding the single AAV packaging limit by employing a dual adeno-associated virus (AAV) strategy to deliver the full-length PCDH15 coding sequence.
We demonstrate the efficacy of this strategy in mouse USH1F models, effectively restoring hearing and balance in these mice.
Importantly, our approach also proves successful in expressing PCDH15 protein in clinically relevant retinal models, including human retinal organoids and non-human primate retina, showing efficient targeting of photoreceptors and proper protein expression in the calyceal processes.
This research represents a major step toward advancing gene therapy for USH1F and the multiple challenges of hearing, balance, and vision impairment.