Summary: According to researchers, a single infusion of HSPCs may halt cellular damage caused by Friedreich’s ataxia.
Source: UCSD.
Researchers at University of California San Diego School of Medicine report that a single infusion of wildtype hematopoietic stem and progenitor cells (HSPCs) into a mouse model of Friedreich’s ataxia (FA) measurably halted cellular damage caused by the degenerative disease.
The findings, published online in the October 25 issue of Science Translational Medicine, suggest a potential therapeutic approach for a disease that currently is considered incurable.
Friedreich’s ataxia is an inherited, degenerative neuromuscular disorder that initially impairs motor function, such as gait and coordination, but can lead to scoliosis, heart disease, vision loss and diabetes. Cognitive function is not affected. The disease is progressively debilitating, and ultimately requires full-time use of a wheelchair. One in 50,000 Americans has FA.
FA is caused by reduced expression of a mitochondrial protein called frataxin (FXN) due to a two mutated or abnormal copies of the FXN gene. In their study, Stephanie Cherqui, PhD, associate professor in the UC San Diego School of Medicine Department of Pediatrics, and colleagues used a transgenic mouse model that expresses two mutant human FXN transgenes, and exhibits the resulting progressive neurological degeneration and muscle weakness.
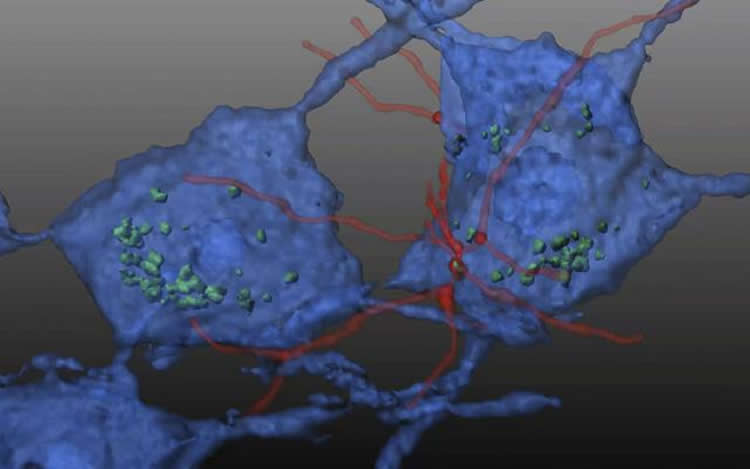
Human hematopoietic stem and progenitor cells (HSPCs), derived from bone marrow, have become a primary vehicle for efforts to replace or regenerate cells destroyed by a variety of diseases. Previous research by Cherqui and colleagues had shown that transplanting wildtype or normal mouse HSPCs resulted in long-term kidney, eye and thyroid preservation in a mouse model of cystinosis, another genetic disorder.
In this study, Cherqui’s team transplanted wildtype HSPCs into an FA mouse model, reporting that the HSPCs engrafted and soon differentiated into macrophages in key regions of the mice’s brain and spinal cord where they appeared to transfer wildtype FXN into deficient neurons and muscle cells.
“Transplantation of wildtype mouse HSPCs essentially rescued FA-impacted cells,” said Cherqui, “Frataxin expression was restored. Mitochondrial function in the brains of the transgenic mice normalized, as did in the heart. There was also decreased skeletal muscle atrophy.”
The scientists note that the mouse model is not perfect mirror of human FA. Disease progression is somewhat different and the precise pathology in mice is not fully known. However, Cherqui said the findings are encouraging and point toward a potential treatment for a disease that currently has none.
Co-authors include: Celine J. Rocca, Spencer M. Goodman, Jennifer N. Dulin, Joseph H. Haquang, Hya Gertsman, Jordan Blondelle, Janell L.M. Smith, and Charles J. Heyser, all at UC San Diego.
Disclosure: Stephanie Cherqui is cofounder, shareholder and a member of both the scientific board and board of directors of GenStem Therapeutics Inc. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
Funding: Funding provided by National Institutes of Health the Cystinosis Research Foundation the Sanford Stem Cell Clinical Center and the California Institute of Regenerative Medicine..
Source: Scott LaFee – UCSD
Publisher: Organized by NeuroscienceNews.com.
Image Source: NeuroscienceNews.com image is credited to Stephanie Cherqui, UC San Diego School of Medicine.
Original Research: Abstract for “Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich’s ataxia” by Celine J. Rocca, Spencer M. Goodman, Jennifer N. Dulin, Joseph H. Haquang, Ilya Gertsman, Jordan Blondelle, Janell L. M. Smith, Charles J. Heyser and Stephanie Cherqui in Science Translational Medicine. Published online October 25 2017 doi:10.1126/scitranslmed.aaj2347
[cbtabs][cbtab title=”MLA”]UCSD “Transplanted Hematopoietic Stem Cells Reverse Damage Caused By Friedreich’s Ataxia.” NeuroscienceNews. NeuroscienceNews, 26 October 2017.
<https://neurosciencenews.com/friedreichs-ataxia-stem-cells-7820/>.[/cbtab][cbtab title=”APA”]UCSD (2017, October 26). Transplanted Hematopoietic Stem Cells Reverse Damage Caused By Friedreich’s Ataxia. NeuroscienceNews. Retrieved October 26, 2017 from https://neurosciencenews.com/friedreichs-ataxia-stem-cells-7820/[/cbtab][cbtab title=”Chicago”]UCSD “Transplanted Hematopoietic Stem Cells Reverse Damage Caused By Friedreich’s Ataxia.” https://neurosciencenews.com/friedreichs-ataxia-stem-cells-7820/ (accessed October 26, 2017).[/cbtab][/cbtabs]
Abstract
Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich’s ataxia
Friedreich’s ataxia (FRDA) is an incurable autosomal recessive neurodegenerative disease caused by reduced expression of the mitochondrial protein frataxin due to an intronic GAA-repeat expansion in the FXN gene. We report the therapeutic efficacy of transplanting wild-type mouse hematopoietic stem and progenitor cells (HSPCs) into the YG8R mouse model of FRDA. In the HSPC-transplanted YG8R mice, development of muscle weakness and locomotor deficits was abrogated as was degeneration of large sensory neurons in the dorsal root ganglia (DRGs) and mitochondrial capacity was improved in brain, skeletal muscle, and heart. Transplanted HSPCs engrafted and then differentiated into microglia in the brain and spinal cord and into macrophages in the DRGs, heart, and muscle of YG8R FRDA mice. We observed the transfer of wild-type frataxin and Cox8 mitochondrial proteins from HSPC-derived microglia/macrophages to FRDA mouse neurons and muscle myocytes in vivo. Our results show the HSPC-mediated phenotypic rescue of FRDA in YG8R mice and suggest that this approach should be investigated further as a strategy for treating FRDA.
“Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich’s ataxia” by Celine J. Rocca, Spencer M. Goodman, Jennifer N. Dulin, Joseph H. Haquang, Ilya Gertsman, Jordan Blondelle, Janell L. M. Smith, Charles J. Heyser and Stephanie Cherqui in Science Translational Medicine. Published online October 25 2017 doi:10.1126/scitranslmed.aaj2347