Summary: A new study identifies neural cells that allow mouse pups to generate ultrasonic calls. Mice that lack the cells are mute. Researchers believe the cries of human babies could depend on similar connections.
Source: Max Delbrück Center.
Genetic study identifies neuronal circuits responsible for ultrasonic calls uttered by mouse pups.
The calls of new-born mice draw the attention of their mother. A group of neuronal cells in the brain stem, which coordinate exhalation and tension of muscles in the larynx is essential for this process. Without these cells, the mice are mute. These are the results of a study by a research team at the Max Delbrück Center in Berlin, which appears in the journal PNAS. The cries of human babies may well depend on similar connections, which could also be impaired in speech disorders.
Almost immediately after birth, mouse pups that are separated from their mother are able to make calls that summon her. The generation of these calls requires vigorous exhalation and the tensioning of laryngeal muscles, which requires the coordinated activity of two muscle groups. This is achieved by neurons in a very old part of the brain, the brainstem, according to a study by Carmen Birchmeier’s lab at the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC). Important contributions to the interdisciplinary study were made by the labs of Jean Champagnat in Paris and Gilles Fortin at the CNRS in Gif sur Yvette.
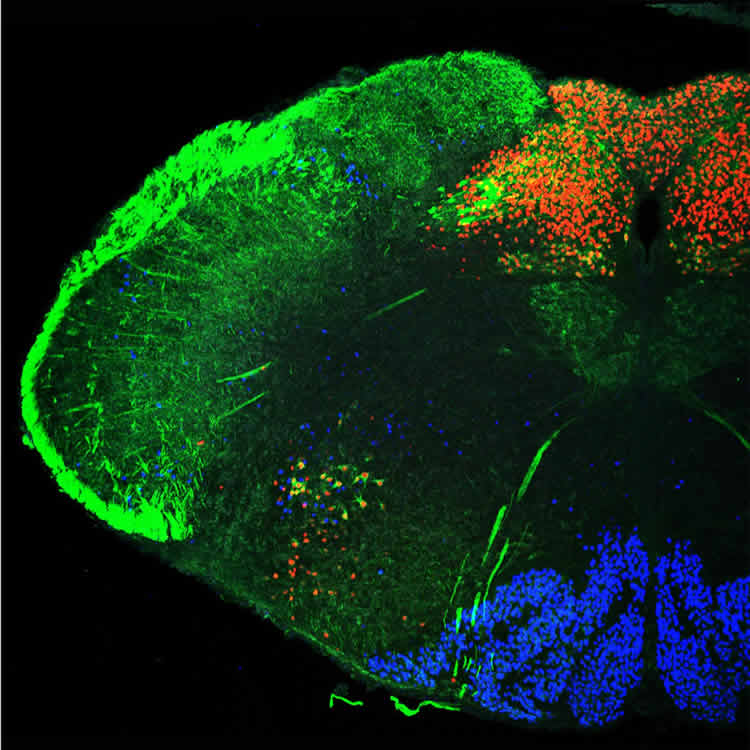
In series of experiments, the researchers have shown that the cells of the nucleus tractus solitarii (NTS) are linked to cells that control tension in the abdominal muscles, enabling vigorous exhalation, and the muscles in the larynx. The nucleus also receives sensory information from the vocal folds, the tongue and the lung. During vocalization, it coordinates sensory inputs and motor outputs. However, if the genes for the transcription factors Olig3 or Tlx3 are mutated, the nerve cells in this particular nucleus cannot mature properly in the fetus. Without it, the pups cannot vocalize after birth.
The mother ignores mute offspring
Newborn mice need to be close to their mother and indicate this to her. As soon as a newborn mouse pup escapes the safety of the nest it emits salvos of four to six calls with a frequency of 75 kHz. These sounds are not audible to the human ear. During each call, the newborn mouse exhales deeply. The mother of the mouse, however, responds immediately: she goes looking for her lost progeny and reunites it with the rest of the litter. Even when she hears recorded ultrasound calls, she sets off in pursuit. If a baby mouse in distress is unable to emit these calls, the mother ignores it.
“We suspect that the calls are an evolutionarily conserved signal that indicates the offsprings’ fitness and health,” Carmen Birchmeier says. “The mute mice are also a model for investigating the importance of vocalization for the interaction between mother and baby,” first-author Luis Hernandez-Miranda says.
Another idea is that the functional faults in the nucleus could be involved in the development and manifestation of speech disorders, which are often seen in patients after strokes, those who have a tumor or are suffering from neurodegenerative diseases.
Source: Annette Tuffs – Max Delbrück Center
Image Source: NeuroscienceNews.com image is credited to Luis Hernandez-Miranda, MDC.
Original Research: Full open access research for “Genetic identification of a hindbrain nucleus essential for innate vocalization” by Luis Rodrigo Hernandez-Miranda, Pierre-Louis Ruffault, Julien C. Bouvier, Andrew J. Murray, Marie-Pierre Morin-Surun, Niccolò Zampieri, Justyna B. Cholewa-Waclaw, Elodie Ey, Jean-Francois Brunet, Jean Champagnat, Gilles Fortin, and Carmen Birchmeier in PNAS. Published online July 31 2017 doi:10.1073/pnas.1702893114
[cbtabs][cbtab title=”MLA”]Max Delbrück Center “How Mice Babies Ensure Mother’s Protection.” NeuroscienceNews. NeuroscienceNews, 31 July 2017.
<mouse-mother-attention-7206/>.[/cbtab][cbtab title=”APA”]Max Delbrück Center (2017, July 31). How Mice Babies Ensure Mother’s Protection. NeuroscienceNew. Retrieved July 31, 2017 from mouse-mother-attention-7206/[/cbtab][cbtab title=”Chicago”]Max Delbrück Center “How Mice Babies Ensure Mother’s Protection.” mouse-mother-attention-7206/ (accessed July 31, 2017).[/cbtab][/cbtabs]
Abstract
Genetic identification of a hindbrain nucleus essential for innate vocalization
Vocalization in young mice is an innate response to isolation or mechanical stimulation. Neuronal circuits that control vocalization and breathing overlap and rely on motor neurons that innervate laryngeal and expiratory muscles, but the brain center that coordinates these motor neurons has not been identified. Here, we show that the hindbrain nucleus tractus solitarius (NTS) is essential for vocalization in mice. By generating genetically modified newborn mice that specifically lack excitatory NTS neurons, we show that they are both mute and unable to produce the expiratory drive required for vocalization. Furthermore, the muteness of these newborns results in maternal neglect. We also show that neurons of the NTS directly connect to and entrain the activity of spinal (L1) and nucleus ambiguus motor pools located at positions where expiratory and laryngeal motor neurons reside. These motor neurons control expiratory pressure and laryngeal tension, respectively, thereby establishing the essential biomechanical parameters used for vocalization. In summary, our work demonstrates that the NTS is an obligatory component of the neuronal circuitry that transforms breaths into calls.
“Genetic identification of a hindbrain nucleus essential for innate vocalization” by Luis Rodrigo Hernandez-Miranda, Pierre-Louis Ruffault, Julien C. Bouvier, Andrew J. Murray, Marie-Pierre Morin-Surun, Niccolò Zampieri, Justyna B. Cholewa-Waclaw, Elodie Ey, Jean-Francois Brunet, Jean Champagnat, Gilles Fortin, and Carmen Birchmeier in PNAS. Published online July 31 2017 doi:10.1073/pnas.1702893114