A UA researcher has provided the strongest evidence yet that it’s possible for brains to fossilize and, in fact, a set of 520-million-year-old arthropod brains have done just that
Science has long dictated that brains don’t fossilize, so when Nicholas Strausfeld co-authored the first ever report of a fossilized brain in a 2012 edition of Nature, it was met with “a lot of flack.”
“It was questioned by many paleontologists, who thought – and in fact some claimed in print – that maybe it was just an artifact or a one-off, implausible fossilization event,” said Strausfeld, a Regents’ professor in UA’s Department of Neuroscience.
His latest paper in Current Biology addresses these doubts head-on, with definitive evidence that, indeed, brains do fossilize.
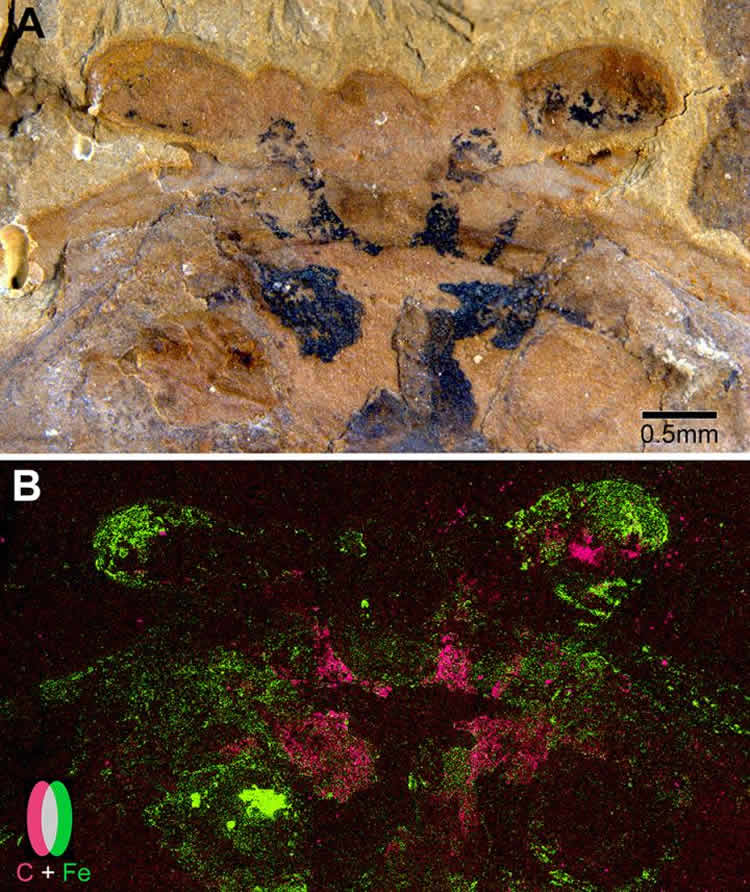
In the paper, Strausfeld and his collaborators, including Xiaoya Ma of Yunnan Key Laboratory for Palaeobiology at China’s Yunnan University and Gregory Edgecombe of the Natural History Museum in London, analyze seven newly discovered fossils of the same species to find, in each, traces of what was undoubtedly a brain.
The species, Fuxianhuia protensa, is an extinct arthropod that roamed the seafloor about 520 million years ago. It would have looked something like a very simple shrimp. And each of the fossils – from the Chengjiang Shales, fossil-rich sites in Southwest China – revealed F. protensa’s ancient brain looked a lot like a modern crustacean’s, too.
Using scanning electron microscopy, the researchers found that the brains were preserved as flattened carbon films, which, in some fossils, were partially overlaid by tiny iron pyrite crystals. This led the research team to a convincing explanation as to how and why neural tissue fossilizes.
In another recent paper in Philosophical Transactions of the Royal Society B, Strausfeld’s experiments uncovered what it likely was about ancient environmental conditions that allowed a brain to fossilize in the first place.
The only way to become fossilized is, first, to get rapidly buried. Hungry scavengers can’t eat a carcass if it’s buried, and as long as the water is anoxic enough – that is, lacking in oxygen – a buried creature’s tissues evade consumption by bacteria as well. Strausfeld and his collaborators suspect F. protensa was buried by rapid, underwater mudslides, a scenario they experimentally recreated by burying sandworms and cockroaches in mud.
This is only step one. Step two, explained Strausfeld, is where most brains would fail: Withstanding the pressure from being rapidly buried under thick, heavy mud.
To have been able to do this, the F. protensa nervous system must have been remarkably dense. In fact, tissues of nervous systems, including brains, are densest in living arthropods. As a small, tightly packed cellular network of fats and proteins, the brain and central nervous system could pass step two, just as did the sandworm and cockroach brains in Strausfeld’s lab.
“Dewatering is different from dehydration, and it happens more gradually,” said Strausfeld, referring to the process by which pressure from the overlying mud squeezes water out of tissues. “During this process, the brain maintains its overall integrity leading to its gradual flattening and preservation. F. protensa‘s tissue density appears to have made all the difference.”
Now that he and his collaborators have produced unassailable evidence that fossilized arthropod brains are more than just a one-off phenomenon, Strausfeld is working to elucidate the origin and evolution of brains over half a billion years in the past.
“People, especially scientists, make assumptions. The fun thing about science, actually, is to demolish them,” said Strausfeld.
Funding: The research was funded by Natural Environment Research Council, Leverhulme Trust, National Natural Science Foundation of China, Yunnan Province of China, Air Force Research Laboratory, and Center for Insect Science University of Arizona.
Source: Emily Litvack – University of Arizona
Image Credit: The image is credited to Strausfeld et al. and Current Biology
Original Research: Full open access research for “Unlocking the early fossil record of the arthropod central nervous system” by Gregory D. Edgecombe, Xiaoya Ma, and Nicholas J. Strausfeld in Philosophical Transactions of the Royal Society B. Published online November 9 2015 doi:10.1098/rstb.2015.0038
Full open access research for “Preservational Pathways of Corresponding Brains of a Cambrian Euarthropod” by Xiaoya Ma, Gregory D. Edgecombe, Xianguang Hou, Tomasz Goral, Nicholas J. Strausfeld in Philosophical Transactions of the Royal Society B. Published online October 29 2015 doi:10.1016/j.cub.2015.09.063
Abstract
Unlocking the early fossil record of the arthropod central nervous system
Extant panarthropods (euarthropods, onychophorans and tardigrades) are hallmarked by stunning morphological and taxonomic diversity, but their central nervous systems (CNS) are relatively conserved. The timing of divergences of the ground pattern CNS organization of the major panarthropod clades has been poorly constrained because of a scarcity of data from their early fossil record. Although the CNS has been documented in three-dimensional detail in insects from Cenozoic ambers, it is widely assumed that these tissues are too prone to decay to withstand other styles of fossilization or geologically older preservation. However, Cambrian Burgess Shale-type compressions have emerged as sources of fossilized brains and nerve cords. CNS in these Cambrian fossils are preserved as carbon films or as iron oxides/hydroxides after pyrite in association with carbon. Experiments with carcasses compacted in fine-grained sediment depict preservation of neural tissue for a more prolonged temporal window than anticipated by decay experiments in other media. CNS and compound eye characters in exceptionally preserved Cambrian fossils predict divergences of the mandibulate and chelicerate ground patterns by Cambrian Stage 3 (ca 518 Ma), a dating that is compatible with molecular estimates for these splits.
“Unlocking the early fossil record of the arthropod central nervous system” by Gregory D. Edgecombe, Xiaoya Ma, and Nicholas J. Strausfeld in Philosophical Transactions of the Royal Society B. Published online November 9 2015 doi:10.1098/rstb.2015.0038
Abstract
Preservational Pathways of Corresponding Brains of a Cambrian Euarthropod
Highlights
•Brain organization of Cambrian arthropod fossils of Fuxianhuia protensa correspond
•Fossils resolve tripartite brain organization, cephalic nerves, and optic neuropils
•SEM and EDX demonstrate that brain traces are carbon films, variably overlaid with pyrite
•Early diagenetic mineralization is not essential for preservation of labile tissues
Summary
The record of arthropod body fossils is traceable back to the “Cambrian explosion,” marked by the appearance of most major animal phyla. Exceptional preservation provides crucial evidence for panarthropod early radiation. However, due to limited representation in the fossil record of internal anatomy, particularly the CNS, studies usually rely on exoskeletal and appendicular morphology. Recent studies [ 1–3 ] show that despite extreme morphological disparities, euarthropod CNS evolution appears to have been remarkably conservative. This conclusion is supported by descriptions from Cambrian panarthropods of neural structures that contribute to understanding early evolution of nervous systems and resolving controversies about segmental homologies [ 4–12 ]. However, the rarity of fossilized CNSs, even when exoskeletons and appendages show high levels of integrity, brought into question data reproducibility because all but one of the aforementioned studies were based on single specimens [ 13 ]. Foremost among objections is the lack of taphonomic explanation for exceptional preservation of a tissue that some see as too prone to decay to be fossilized. Here we describe newly discovered specimens of the Chengjiang euarthropod Fuxianhuia protensa with fossilized brains revealing matching profiles, allowing rigorous testing of the reproducibility of cerebral structures. Their geochemical analyses provide crucial insights of taphonomic pathways for brain preservation, ranging from uniform carbon compressions to complete pyritization, revealing that neural tissue was initially preserved as carbonaceous film and subsequently pyritized. This mode of preservation is consistent with the taphonomic pathways of gross anatomy, indicating that no special mode is required for fossilization of labile neural tissue.
“Preservational Pathways of Corresponding Brains of a Cambrian Euarthropod” by Xiaoya Ma, Gregory D. Edgecombe, Xianguang Hou, Tomasz Goral, Nicholas J. Strausfeld in Philosophical Transactions of the Royal Society B. Published online October 29 2015 doi:10.1016/j.cub.2015.09.063