Neuroscientists have perfected a chemical-genetic remote control for brain circuitry and behavior. This evolving technology can now sequentially switch the same neurons – and the behaviors they mediate – on-and-off in mice, say researchers funded by the National Institutes of Health. Such bidirectional control is pivotal for decoding the brain workings of complex behaviors. The findings are the first to be published from the first wave of NIH grants awarded last fall under the BRAIN Initiative.
“With its new push-pull control, this tool sharpens the cutting edge of research aimed at improving our understanding of brain circuit disorders, such as schizophrenia and addictive behaviors,” said NIH director Francis S. Collins, M.D., Ph.D.
Bryan Roth, M.D., Ph.D. , of the University of North Carolina, Chapel Hill; Michael Krashes, Ph.D. , of NIH’s National Institute of Diabetes and Digestive and Kidney Diseases; and colleagues, debut the second generation of the tool, called DREADD – Designer Receptors Exclusively Activated by Designer Drugs – on April 30, 2014 in the journal Neuron.
DREADD 2.0 improves on a widely-adopted technology developed by Roth, a grantee of NIH’s National Institute of Mental Health, and colleagues, over the past decade. It achieves remote control by introducing a synthetic brain chemical messenger system that integrates with the workings of naturally-occurring systems.
Researchers genetically-engineer mice to have brains containing what they dub “designer receptors” in specific circuits. These are synthetic proteins on the surface of neurons that can only be activated by a matching synthetic chemical that otherwise has no biological effect – like a lock that can only be opened by a unique key. When the “designer drug” binds to its receptor, depending on its programming, it either triggers or blocks neuronal activity, thus giving researchers experimental control over the animal’s brain circuits and behaviors.
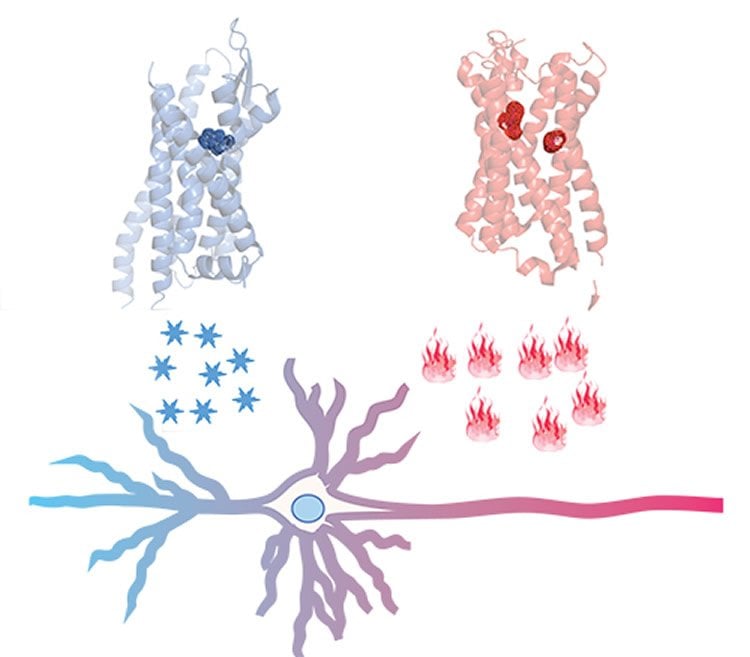
Early iterations of DREADD could only control activity in one direction – on or off – in the same population of cells. DREADD 2.0 takes advantage of properties offered by a particular type of receptor, paired with a biologically inert chemical that binds to it, to add bidirectional control. Coupled with an existing DREADD, it can be used experimentally to probe circuitry of a broad range of behaviors via sequential, on-and-off control of neurons. It’s like having two sets of locks with their own unique keys – one triggering “on,” the other turning “off.” For example, the researchers demonstrated how the improved DREAD toolkit can bi-directionally control animals’ movement and feeding behaviors.
Since DREADD effects last about an hour – as opposed to milliseconds with an alternative optical-genetic technology – it may be the tool of choice for studies of behaviors requiring prolonged control of circuitry and/or minimal invasiveness.
Funding: This research was fundedby the NIH – grants MH105892, DA017204, DA035764, DK075087, DK075089, AA019454, AA17668, AA020911, AA02228001, AA018335, AA021312.
Source: Jules Asher – NIH/NIMH
Image Source: The image is credited to Bryan Roth, Ph.D., University of North Carolina
Original Research: Abstract for “A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior” by Eyal Vardy, J. Elliott Robinson, Chia Li, Reid H.J. Olsen, Jeffrey F. DiBerto, Patrick M. Giguere, Flori M. Sassano, Xi-Ping Huang, Hu Zhu, Daniel J. Urban, Kate L. White, Joseph E. Rittiner, Nicole A. Crowley, Kristen E. Pleil, Christopher M. Mazzone, Philip D. Mosier, Juan Song, Thomas L. Kash, C.J. Malanga, Michael J. Krashes, and Bryan L. Roth in Neuron. Published online April 30 2015 doi:10.1016/j.neuron.2015.03.065
Abstract
A New DREADD Facilitates the Multiplexed Chemogenetic Interrogation of Behavior
Highlights
•Structure-guided approach for κ-opioid receptor (KOR)-DREADD (KORD) design
•KORD is selectively activated by salvinorin B, and not by endogenous opioids
•KORD robustly silenced multiple neuronal subtypes
•Inhibitory KORD combined with excitatory hM3Dq for multiplexed behavioral control
Summary
DREADDs are chemogenetic tools widely used to remotely control cellular signaling, neuronal activity, and behavior. Here we used a structure-based approach to develop a new Gi-coupled DREADD using the kappa-opioid receptor as a template (KORD) that is activated by the pharmacologically inert ligand salvinorin B (SALB). Activation of virally expressed KORD in several neuronal contexts robustly attenuated neuronal activity and modified behaviors. Additionally, co-expression of the KORD and the Gq-coupled M3-DREADD within the same neuronal population facilitated the sequential and bidirectional remote control of behavior. The availability of DREADDs activated by different ligands provides enhanced opportunities for investigating diverse physiological systems using multiplexed chemogenetic actuators.
“A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior” by Eyal Vardy, J. Elliott Robinson, Chia Li, Reid H.J. Olsen, Jeffrey F. DiBerto, Patrick M. Giguere, Flori M. Sassano, Xi-Ping Huang, Hu Zhu, Daniel J. Urban, Kate L. White, Joseph E. Rittiner, Nicole A. Crowley, Kristen E. Pleil, Christopher M. Mazzone, Philip D. Mosier, Juan Song, Thomas L. Kash, C.J. Malanga, Michael J. Krashes, and Bryan L. Roth in Neuron. Published online April 30 2015 doi:10.1016/j.neuron.2015.03.065






