Summary: Researchers believe their findings may provide a new path to the study of Alzheimer’s disease and its cause.
Source: Stony Brook University.
Accumulating amounts of amyloid, which is a fragment of a larger protein, in the brain have been associated with the development of dementia, including Alzheimer’s disease. Now a team of neuroscience and biochemistry researchers at Stony Brook University have made a novel discovery that illustrates for the first time the difference between amyloid buildup in brain blood vessels and amyloid buildup around brain neurons. Their findings, which may provide a new path to research on Alzheimer’s disease and its cause, will be published November 21 in Nature Communications.
Lead investigator William Van Nostrand, PhD, a Professor in the Department of Neurosurgery, says the findings stem from collaborative work with Steven Smith, PhD, a Professor in the Department of Biochemistry & Cell Biology. They, along with colleagues, mapped out the structural signature of amyloid that accumulates in brain blood vessels and compared it to the known structure of amyloid that accumulate in plaque around brain neurons.
The team found that the subunits of the amyloid that accumulates in vessels line up uniquely and in alternating patterns, which presents in a near opposite pattern of amyloid buildup in plaque around neurons.
“This discovery may help guide us to the development of a new diagnostic tool or therapeutic intervention for dementia patients who display this vessel pathology,” summarized Dr. Van Nostrand.
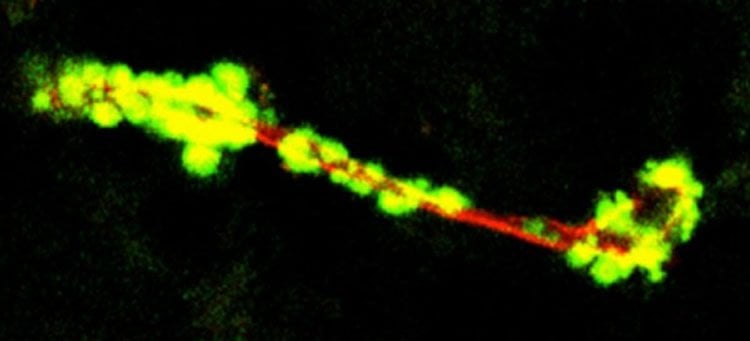
They hypothesize that the unique structure of this brain blood vessel amyloid could promote different pathological responses, ie, inflammation, which likely contributes differently to cognitive impairment and dementia than neuron amyloid.
Source: William Van Nostrand, PhD – Stony Brook University
Image Source: NeuroscienceNews.com image is adapted from the Stony Brook press release.
Original Research: Full open access research for “Cerebral vascular amyloid seeds drive amyloid β-protein fibril assembly with a distinct anti-parallel structure” by Feng Xu, Ziao Fu, Sharmila Dass, AnnMarie E. Kotarba, Judianne Davis, Steven O. Smith & William E. Van Nostrand in Nature Communications. Published online November 21 2016 doi:10.1038/ncomms13527
[cbtabs][cbtab title=”MLA”]Stony Brook University. “Unique Structure Of Brain Blood Vessel Amyloid Latest Clue To Alzheimer’s Development?.” NeuroscienceNews. NeuroscienceNews, 21 November 2016.
<https://neurosciencenews.com/alzheimers-amyloid-blood-vessel-5567/>.[/cbtab][cbtab title=”APA”]Stony Brook University. (2016, November 21). Unique Structure Of Brain Blood Vessel Amyloid Latest Clue To Alzheimer’s Development?. NeuroscienceNews. Retrieved November 21, 2016 from https://neurosciencenews.com/alzheimers-amyloid-blood-vessel-5567/[/cbtab][cbtab title=”Chicago”]Stony Brook University. “Unique Structure Of Brain Blood Vessel Amyloid Latest Clue To Alzheimer’s Development?.” https://neurosciencenews.com/alzheimers-amyloid-blood-vessel-5567/ (accessed November 21, 2016).[/cbtab][/cbtabs]
Abstract
Cerebral vascular amyloid seeds drive amyloid β-protein fibril assembly with a distinct anti-parallel structure
Cerebrovascular accumulation of amyloid β-protein (Aβ), a condition known as cerebral amyloid angiopathy (CAA), is a common pathological feature of patients with Alzheimer’s disease. Familial Aβ mutations, such as Dutch-E22Q and Iowa-D23N, can cause severe cerebrovascular accumulation of amyloid that serves as a potent driver of vascular cognitive impairment and dementia. The distinctive features of vascular amyloid that underlie its unique pathological properties remain unknown. Here, we use transgenic mouse models producing CAA mutants (Tg-SwDI) or overproducing human wild-type Aβ (Tg2576) to demonstrate that CAA-mutant vascular amyloid influences wild-type Aβ deposition in brain. We also show isolated microvascular amyloid seeds from Tg-SwDI mice drive assembly of human wild-type Aβ into distinct anti-parallel β-sheet fibrils. These findings indicate that cerebrovascular amyloid can serve as an effective scaffold to promote rapid assembly and strong deposition of Aβ into a unique structure that likely contributes to its distinctive pathology.
“Cerebral vascular amyloid seeds drive amyloid β-protein fibril assembly with a distinct anti-parallel structure” by Feng Xu, Ziao Fu, Sharmila Dass, AnnMarie E. Kotarba, Judianne Davis, Steven O. Smith & William E. Van Nostrand in Nature Communications. Published online November 21 2016 doi:10.1038/ncomms13527